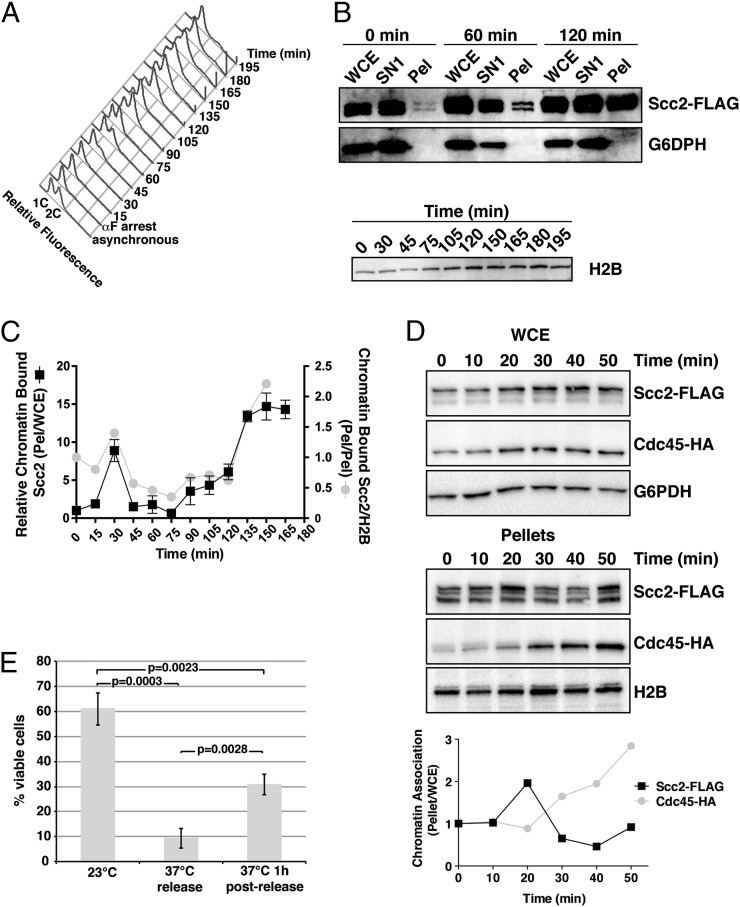
Fig. 1.
Scc2 chromatin association is biphasic. (A) DNA histograms of propidium iodide-stained (1891-32C) cells are shown in an asynchronous population and following αF-arrest and release into fresh media at 23 °C in 15-min intervals. Pre- and post-DNA replication DNA contents (1C and 2C, respectively) are indicated. (B) FLAG and G6PDH immunoblots of WCE, SN1, and pellet fractions and H2B immunoblots of pellet fractions are shown for a subset of the time course samples taken in A. (C) Scc2 protein levels in pellets were analyzed relative to their levels in WCEs (Pel:WCE, black squares), as determined by using semiquantitative immunoblotting, and this ratio was set equal to 1 for the 0 min time point. Subsequent time points were calculated relative to 0 min. Levels of Scc2-FLAG in time course pellet fractions were also normalized to chromatin-bound H2B (gray circles), as an additional control. (D) Scc2-FLAG Cdc45-HA (PMY715) cells were released from an αF arrest and sampled by chromatin fractionation at the time of release (0 min) and at 10-min intervals. FLAG, HA, G6PDH, and H2B immunoblots of WCE and pellet fractions are shown. Quantitation of ratios of Scc2 and Cdc45 in pellets as a function of their amounts in WCE is shown. (E) Viabilities of scc2-4 (1875-39B) cells under the indicated release conditions are shown with error bars indicating SD, n = 3. Student t tests determined significance, P.