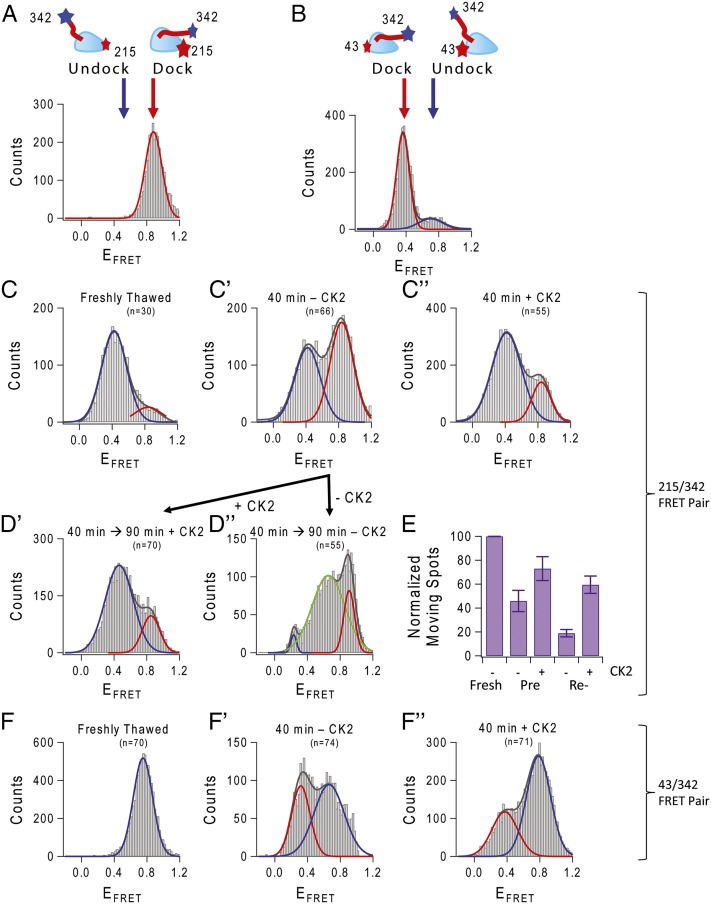
Fig. 5.
Conformational change controlling NL position is altered by CK2. The relative position of the NL and MD can be monitored by Cy3–Cy5 single dye-pair FRET. Using 43/342 and 215/342 cysteine pairs introduced to monomeric Cys-light kinesin (see cartoon in A and B), we can monitor movement of the NL relative to the MD. (A and B) Binding labeled freshly thawed kinesin to axoneme MT in the presence of AMP-PNP served as a control to show docking of the NL. In all histograms, red represents the Gaussian fit for the docked signal, and blue is the fit for the undocked state. (C) Movement of the NL visualized using kinesin labeled at 215/342 cysteines attached to coverslip in the absence of microtubule. Freshly thawed kinesin began in the undocked position. (C′) After aging at 30 °C, the FRET efficiency distribution changed to one that had a significant contribution from a second peak, localized at ∼0.8 FRET efficiency (compare with docked signal in A). (C″) This docked-like conformation was prevented with CK2 treatment. (D′) Not only did CK2 prevent the docking of the NL, CK2 treatment could reverse docking of the NL. CK2 aged an additional 90 min on ice showed rescue of the docked NL. (D″) Kinesin left untreated had a different distribution of peak FRET efficiencies, with a new peak appearing at EFRET ∼0.6. (E) FRET data were validated with fluorescently labeled dimeric single kinesins moving on axoneme MT. Landing and movement events were counted in CK2-preincubated treated and untreated kinesin, as well as CK2-reactivated treated and untreated kinesin. (F) Kinesin labeled at 43/342 cysteines, freshly thawed, has an undocked EFRET peak at ∼0.8 (compare with docked state in B). (F′) Aged 43/342 kinesin shifted into the docked-like peak and was reversed with treatment by CK2 (F″).