Abstract
The role of integrated 18F-2-fluoro-2-deoxy-D-glucose positron emission tomography computed tomography (PET-CT) is uncertain in gallbladder cancer. The aim of this study was to show the role of PET-CT in gallbladder cancer patients. Fifty-three patients with gallbladder cancer underwent preoperative computed tomography (CT) and PET-CT scans. Their medical records were retrospectively reviewed. Twenty-six patients underwent resection. Based on the final outcomes, PET-CT was in good agreement (0.61 to 0.80) with resectability whereas CT was in acceptable agreement (0.41 to 0.60) with resectability. When the diagnostic accuracy of the predictions for resectability was calculated with the ROC curve, the accuracy of PET-CT was higher than that of CT in patients who underwent surgical resection (P=0.03), however, there was no difference with all patients (P=0.12). CT and PET-CT had a discrepancy in assessing curative resection in nine patients. These consisted of two false negative and four false positive CT results (11.3%) and three false negative PET-CT results (5.1%). PET-CT was in good agreement with the final outcomes compared to CT. As a complementary role of PEC-CT to CT, PET-CT tended to show better prediction about resectability than CT, especially due to unexpected distant metastasis.
Graphical Abstract

Keywords: Gallbladder Neoplasms; Positron-Emission Tomography; Tomography, X-Ray Computed; Diagnosis
INTRODUCTION
Biliary tract cancer including gallbladder cancer (GBC) is the eighth most common cancer in Korea, 2010 (1). Among these, GBC that arises from the epithelium of the gallbladder is the most common and aggressive cancer of the biliary tract. Since complete surgical resection is the only curative therapy, a precise initial evaluation for surgical resectability is mandatory; however, GBC has a frequent regional metastasis at presentation (2). Therefore, it is sometimes very difficult to select patients who will be good candidates for curative surgical resection because there can be occasionally ambiguous lymph node metastasis on the work-up with conventional radiological imaging. Additionally, there is a risk for initially unsuspected distant metastasis since GBC has more potential risk for synchronous distant metastasis at initial diagnosis than hilar cholangiocarcinoma (3).
Positron emission tomography (PET) using 18F-2-fluoro-2-deoxy-D-glucose (18F-FDG) can show malignant tumors since cancer cells utilize more glucose than normal tissue cells. Thus, it can provide physiological or metabolic information rather than anatomical information on tumors. However, non-anatomical visualization features have some limitations such as low-resolution images and poor anatomical localization. As a result, a correlation between PET and other conventional techniques like computed tomography (CT) or magnetic resonance imaging must be considered for a correct diagnosis. To overcome these drawbacks, the combination of a PET scanner with a multi-detector row helical CT-Integrated positron emission tomography and computed tomography (PET-CT) was proposed (4). The advantages of this new technique have been established for many solid cancers (5). However, there are only a few reports on PET-CT for biliary tract tumors and most studies were done without differentiation between the gallbladder and other biliary tracts due to the relative low incidence of these diseases (6, 7). Therefore, the aim of this study was to evaluate the efficacy of PET-CT compared to CT, especially for its clinical role in assessing curative surgical resection in GBC.
MATERIALS AND METHODS
Patients
From October 2003 to November 2010, 59 patients who were diagnosed with GBC underwent PET-CT as an initial work-up for an assessment of surgical resectability at Seoul National University Hospital. Diagnosis of GBC was based on suspicious radiological imaging and pathological confirmation by surgical specimens or by the results of a biopsy. Among these, 6 patients who underwent the CT with a multi-detector using less than eight slices were excluded in this study due to low-resolution. As a result, 53 patients were included in the study and their medical records and radiological findings were reviewed, retrospectively. Because Seoul National University Hospital is a referral center, all patients had already received CT in other hospital. In some patients, however, the quality of CT in other hospital was usually not satisfactory to decide the surgical resectability. As a result, 42 (78.2%) patients underwent multi-detector CT in our center again. In contrast, the quality of initial CT was satisfactory in 11 (20.8%) patients who did not receive CT in our center. The interval between CT and PET-CT was less than 2 weeks.
CT and PET-CT
Before the CT examination, patients fasted for at least eight hours. Contrast material enhancement was achieved by intravenous administration of non-ionic contrast material with a power injector. For greater detailed images with a shorter examination time, conventional CT images were acquired by eight or more detector CT scanners as follows: Brilliance 64, Definition AS, MX 8000 (Philips Medical Systems, Best, Netherlands), SOMATOM Emotion, SOMATOM Sensation 16/64 (Siemens Medical Solutions, Erlangen, Germany), or LightSpeed VCT/16/Plus/Ultra (General Electric Healthcare, Milwaukee, Wisconsin, USA). 18F-FDG PET was done with 2 PET-CT scanners, the Biograph 40 (Siemens Medical Solutions) or Gemini (Philips Medical Systems). After fasting for at least six hours, patients were injected with 5.2 MBq/kg of 18F-FDG and the images were acquired one hour later. CT scan was done for attenuation correction, and afterwards, emission scan was done from the skull base to the proximal thigh. Images were reconstructed using an iterative algorithm (ordered subset expectation maximization). Images were blindly interpreted by 2 experienced nuclear medicine physicians without information of the CT scan and conclusions were made by consensus. As a semi-quantitative analysis of the images, the standardized uptake value (SUV) of the suspicious areas was calculated as follows:
| SUV=(injected dose corrected by disintegration/activity concentration)/(injected dose/body weight) |
All the maximum SUVs in the gallbladder, lymph node, and distant metastasis were calculated. Detection of primary tumor, lymph node, and distant metastasis were dichotomized as either positive or negative in CT and PET-CT.
Definition of stage and resectability
According to the American Joint Committee on Cancer staging manual seventh edition (8), N1 lymph node is a regional lymph node along the cystic duct, common bile duct, hepatic artery, and/or portal vein and N2 lymph node is a regional lymph node along the periaortic, pericaval, superior mesenteric artery, and/or celiac artery. In addition, lymph node in the hepaticoduodenal ligament distribution is considered as N2 lymph node. With CT scan, lymph nodes more than 10 mm in diameter were usually considered pathological. In addition, they were also considered as tumor involvements if other signs of malignancy were encountered, e.g. grouping of nodes, central necrosis, shape or pathological contrast material enhancement. With PET-CT scans, metastatic lymph nodes were considered if the FDG uptake of lymph node was more than that of the surrounding tissue in the visual analysis in addition to the signs of malignancy as above mentioned. M1 refers to distant metastasis including intrahepatic metastasis. Radiological staging was assessed by CT and PET-CT, respectively. The final outcomes of lymph node, distant metastasis, and resectability were decided by pathological results or clinical follow-up with serial radiological imaging more than 3 months from the initial work-up. Stage IVB GBC (Any T stage with N2 lymph node or M1 metastasis) was recognized as an unresectable tumor.
Statistics
Statistical analysis was done with statistical software (SPSS version 19.0 for Windows, SPSS Inc, Chicago, IL, USA; MedCalc version 11.5.0.0, MedCalc Software, Mariakerke, Belgium). The agreements for lymph node, distant metastasis, and resectability between the final outcomes and CT or PET-CT were analyzed by kappa analysis (Cohen's kappa, κ). Kappa values above 0.8, values from 0.61 to 0.80, from 0.41 to 0.60, from 0.21 to 0.40, and values below 0.20 were considered excellent, good, acceptable, regular, and fair, respectively (9). Sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) for CT and PET-CT were calculated and compared by chi square test, Fisher's exact test, or McNemar test. Two-sided P values less than 0.05 were considered statistically significant. The diagnostic accuracy of the prediction of resectability was calculated with area under curve (AUC) using receiver operating characteristic (ROC) analysis.
Ethics statement
The study was approved by the institutional review board of Seoul National University Hospital (IRB number H-1105-113-364). Informed consent was waived by the IRB.
RESULTS
Baseline characteristics
The mean age was 62.4 ± 10.0 yr (range, 38-82) and males comprised 47.2% (25 of 53) of the study population. Twenty-six patients (49.1%) underwent surgery as follows: extended cholecystectomy (n = 8, 15.1%) which consisted of wedge resection of the liver at the gallbladder bed and regional lymphadenectomy, open cholecystectomy with or without liver resection (n = 7, 13.2%), palliative cholecystectomy (n = 7, 13.2%), laparoscopic cholecystectomy (n = 3, 5.7%), and extended right lobectomy of liver (n=1, 3.8%) because of two hepatic metastases (size: 2.5 × 2.0 × 1.7 cm and 0.5 × 0.5 × 0.5 cm). The mean level of the maximum SUV on PET-CT for the gallbladder, lymph node, and distant metastatic organ were 7.7 ± 5.1, 6.2 ± 2.9, and 6.4 ± 2.8, respectively. The various stages according to CT, PET-CT, and pathology are presented in Table 1. Due to the limitations of the assessment method for primary tumor invasion (T) with CT or PET-CT although usual resolution of CT was better than that of PET-CT, stage I and II were classified into the same category as stage under II.
Table 1.
Stages of subjected patients according to CT, PET-CT, and final outcomes
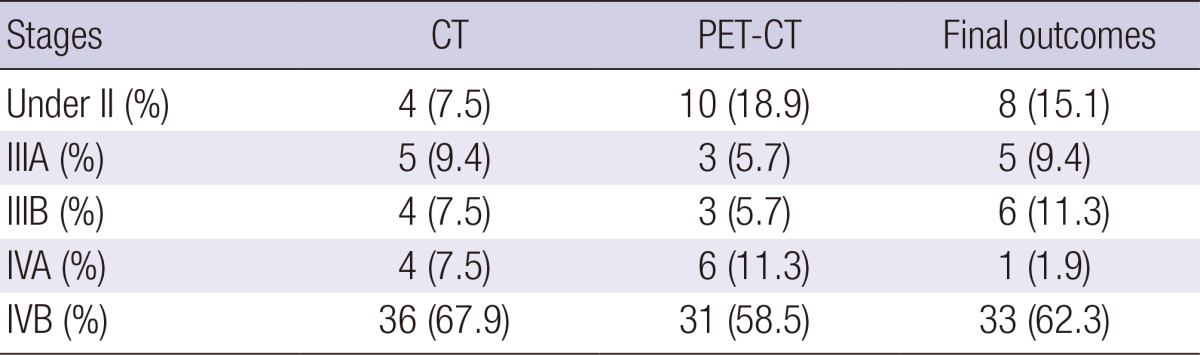
CT, computerized tomography; PET-CT, integrated positron emission tomography and computed tomography.
Agreements for lymph node, distant metastasis and resectability
Results of the analyses about the agreements for resectability between the final outcomes and CT or PET-CT are presented in Table 2. Contrary to CT having an acceptable agreement (0.40 < kappa value ≤ 0.60) with resectability in terms of the final outcomes, PET-CT was in good agreement (0.60 < kappa value ≤ 0.80) with resectability. Between CT and PET-CT, the kappa value (mean ± SD) for resectability was 0.64±0.11, respectively (P value < 0.001; data not shown).
Table 2.
Agreement analysis for resectability between final outcomes and CT or PET-CT
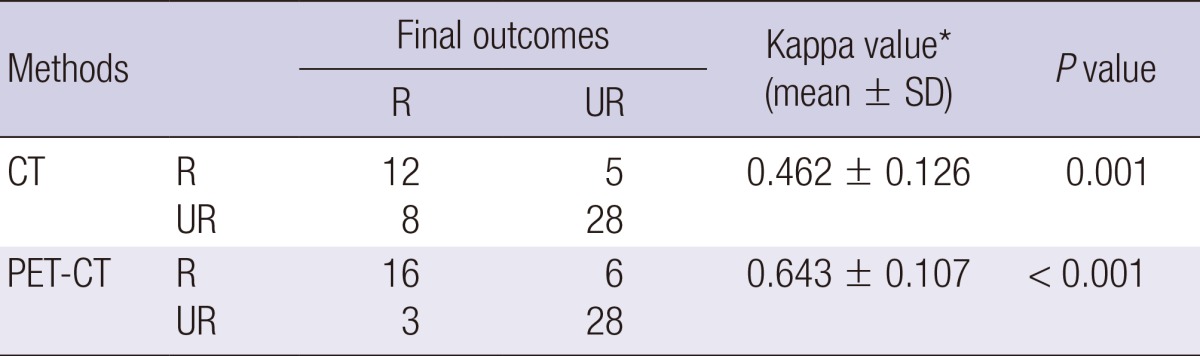
*Kappa values are interpreted as follows: less than 0.20, fair; 0.21 to 0.40, regular; 0.41 to 0.60, acceptable; 0.61 to 0.80, good; above 0.8, excellent. CT, computerized tomography; PET-CT, integrated positron emission tomography and computed tomography; R, resectable; UR, unresectable.
Based on these results, both CT and PET-CT eventually assessed the resectability as the same in 44 patients (38 cases of correct assessment and 6 cases of incorrect assessment for both CT and PET-CT) and different in 9 patients. Of the 6 cases with both incorrect assessment, there were 3 patients with overcalling disease (suspected disease really was not present) and 3 patient with undercalling disease (unsuspected disease was in fact present) of CT and PET-CT. Among the 3 patients with overcalling disease, there were 2 patients with the periaortic lymph nodes, pericaval lymph nodes, and lymph nodes along the superior mesentery artery which showed maximum SUV from 2.1 to 3.4; however, they were not true metastatic nodes. And one of these two patients had a hypermetabolic (maximum SUV = 3.4) lesion in distal esophagus which was not present, too. Similarly, the other patient with overcalling disease had retroperitoneal lymph nodes which showed maximum SUV 9.4; however, there was no metastasis in this lesion by extended cholecystectomy with pylorus preserving pancreaticoduodenectomy. By contrast, there were 3 patients with actually undercalling disease. One of these patients had the periaortic and pericaval lymph nodes which were interpreted as indeterminate nodes because maximum SUV were less than 2.0; however, they were metastatic nodes, eventually. And other 2 patients had peritoneal seeding which was not detected by two methods.
Especially in 12 patients without nodal or distant metastasis on CT (TxN0M0), there were eventually 3 metastatic patients who had peritoneal seeding (2 patients) and left mediastinal lymph node metastasis (1 patient). Among these, PET-CT could preoperatively find only one patient with mediastinal lymph node metastasis.
Comparison of CT and PET-CT for resectability
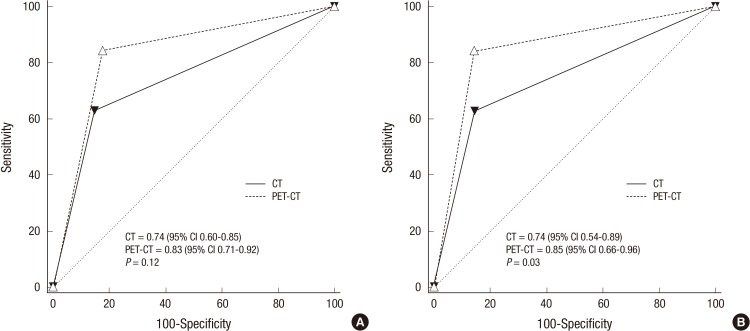
When the diagnostic accuracy of the predictions for resectability was calculated with the ROC curve, PET-CT failed to show a better result than CT (P=0.12, Fig. 1A). However, the accuracy of PET-CT was significantly higher than that of CT in 26 patients who underwent surgical resection (P=0.03, Fig. 1B).
Fig. 1.
Graph shows receiver operating characteristic curves for resectability. (A) There is no difference of receiver operating characteristic curves between 18F-2-fluoro-2-deoxy-D-glucose positron emission tomography (dashed curve) and computed tomography (solid curve) for resectability with all 53 patients. (B) Receiver operating characteristic curves of 18F-2-fluoro-2-deoxy-D-glucose positron emission tomography and computed tomography (dashed curve) is better than those of computed tomography (solid curve) for resectability with 26 patients who underwent surgical resection.
Among 9 patients with different assessment by CT and PET-CT, the assessment by PET-CT for curative surgical resection was correct in 6 cases (4 cases of false positive [FP] and 2 cases of false negative [FN] assessment with CT) and incorrect in 3 cases (all 3 cases were FN with PET-CT) (Table 3).
Table 3.
Results of cases which showed discrepancies of surgical resectability between CT and PET-CT
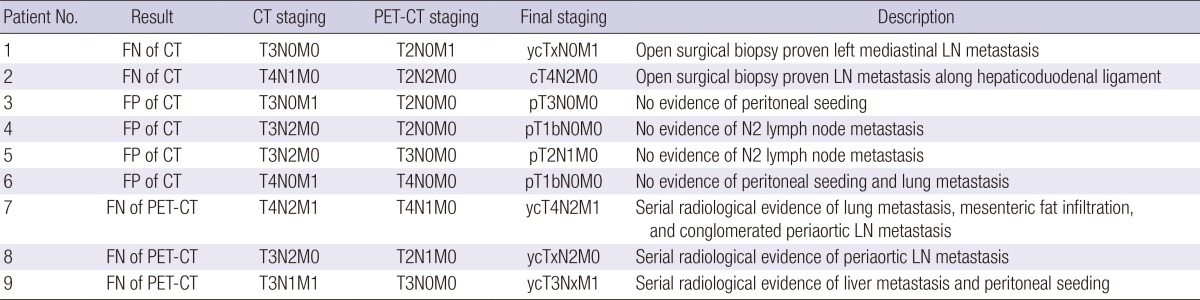
CT, computerized tomography; PET-CT, integrated positron emission tomography and computed tomography; FP, false positive; FN, false negative; T, primary tumor staging; N, lymph node staging; M, distant metastasis staging; LN, lymph node.
DISCUSSION
Although both GBC and bile duct cancer, which are two representative cancers in the biliary tract, are often diagnosed with a locoregional disseminated status, the role of 18F-FDG PET or 18F-FDG PET-CT, which has been proven in a variety of cancers (5, 10), has not been established in biliary tract cancer mainly due to limited evidence from few studies (7, 11, 12, 13, 14). Moreover, although GBC showed different recurrence patterns from hilar cholangiocarcinoma after potentially curative surgical resection such as earlier recurrence and higher involvement of a distal site (3), the usefulness of PET or PET-CT was rarely reported for GBC (15, 16). In the present study, the aim was to assess the clinical usefulness of 18F-FDG PET-CT, especially in terms of curative surgical resection.
In this study, about 85% of patients had advanced stages of GBC beyond stage IIIA including more than half of the cases with stage IVB. It suggests that most patients underwent PET-CT for an assessment of curative surgical resection because surgical resection was the only curative treatment. In this respect, the evaluation focused on N2 lymph node and distant metastasis because T stage has a limited decisive role in curative surgical resection. Additionally, PET-CT has been known to be not superior to conventional contrast enhanced CT in the diagnosis of primary biliary tumors (7, 13, 14). Actually, there were two patients in which PET-CT failed to detect the primary tumor in those studies.
In this study, PET-CT was in good agreement with the final outcomes for surgical resectability while CT was in acceptable agreement with the final outcomes. In addition, the AUC of PET-CT in patients who underwent surgical resection was greater than that of CT although there was no significant difference of AUC from PET-CT and CT with all patients. Considering that several studies have consistently reported a large value of AUC from PET-CT for unsuspected distant metastasis (7, 13, 14, 17), the results of our study suggested that PET-CT has a complementary role to CT.
Based on a comprehensive review, there were eventually 9 discrepant cases for curative surgical resection between the two tests, which consisted of 4 FP and 2 FN results of CT, and 3 FN results of PET-CT. When these results were analyzed, both tests showed a limited ability for an assessment of T staging. Therefore, they assessed 2 cases with pT1b as T3 or T4.
CT had a limitation in assessing N2 lymph node with a size lower than 1 cm or with ambiguous morphology between true metastasis and reactive changes. In addition, it was sometimes too sensitive to assess focal peritoneal seeding with or without small amounts of ascites and especially lacked the ability to find unsuspected distant metastasis, which was in agreement with a previous report (14). On the other hand, PET-CT had a limited ability to assess small metastatic nodules in the lung, liver, or peritoneum that were less than 5 mm in size (11). Additionally, it could not assess lymph nodes, which had low 18F-FDG uptake compared to their size due to internal necrosis or conglomeration.
Among 12 patients without nodal or distant metastasis on CT in this study, 3 patients had eventually metastatic cases and PET-CT could preoperatively find one patient who had left mediastinal lymph node metastasis. Considering that the practical role of the two tests is not exclusive but complementary, PET-CT would be worthy in such cases with exceptional metastasis. However, it should be considered that it also showed a limited usefulness in two patients with peritoneal metastasis.
This study has some limitations. First, this was a retrospective study. As a result, the result of our study only showed the complementary role of PET-CT without statistical significance. Second, various CT scanners were used compared to only two kinds of PET-CT scanners. Third, the majority of the patients were stage IV. Therefore, the results of this study are more appropriate to patients with advanced disease. However, this study is the first report about PET-CT for GBC and suggested the complementary role of PET-CT with GBC, especially in curative surgical resectability.
In conclusion, PET-CT is in good agreement with the final outcomes compared to CT which is in acceptable agreement. As a complementary role of PEC-CT to CT, PET-CT tends to show better prediction about resectability than CT with borderline significance, especially due to unexpected distant metastasis. Further study about the actual role of PET-CT in GBC is necessary.
Footnotes
There is no research grant or funding with respect to this manuscript.
The authors declared no potential conflicts of interest or source of funding with respect to this manuscript.
References
- 1.National Cancer Control Institute. National Cancer Registration and Statistics in Korea 2010. [accessed on 28 Ferbruary 2014]. Available at http://ncc.re.kr/english/infor/kccr.jsp.
- 2.Matsumoto Y, Fujii H, Aoyama H, Yamamoto M, Sugahara K, Suda K. Surgical treatment of primary carcinoma of the gallbladder based on the histologic analysis of 48 surgical specimens. Am J Surg. 1992;163:239–245. doi: 10.1016/0002-9610(92)90109-5. [DOI] [PubMed] [Google Scholar]
- 3.Jarnagin WR, Ruo L, Little SA, Klimstra D, D'Angelica M, DeMatteo RP, Wagman R, Blumgart LH, Fong Y. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98:1689–1700. doi: 10.1002/cncr.11699. [DOI] [PubMed] [Google Scholar]
- 4.Hany TF, Steinert HC, Goerres GW, Buck A, von Schulthess GK. PET diagnostic accuracy: improvement with in-line PET-CT system: initial results. Radiology. 2002;225:575–581. doi: 10.1148/radiol.2252011568. [DOI] [PubMed] [Google Scholar]
- 5.Antoch G, Saoudi N, Kuehl H, Dahmen G, Mueller SP, Beyer T, Bockisch A, Debatin JF, Freudenberg LS. Accuracy of whole-body dual-modality fluorine-18-2-fluoro-2-deoxy-D-glucose positron emission tomography and computed tomography (FDG-PET/CT) for tumor staging in solid tumors: comparison with CT and PET. J Clin Oncol. 2004;22:4357–4368. doi: 10.1200/JCO.2004.08.120. [DOI] [PubMed] [Google Scholar]
- 6.Lee JY, Kim HJ, Yim SH, Shin DS, Yu JH, Ju DY, Park JH, Park DI, Cho YK, Sohn CI, et al. Primary tumor maximum standardized uptake value measured on 18F-fluorodeoxyglucose positron emission tomography-computed tomography is a prognostic value for survival in bile duct and gallbladder cancer. Korean J Gastroenterol. 2013;62:227–233. doi: 10.4166/kjg.2013.62.4.227. [DOI] [PubMed] [Google Scholar]
- 7.Lee SW, Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, Jeon WK, Kim BI. Clinical usefulness of 18F-FDG PET-CT for patients with gallbladder cancer and cholangiocarcinoma. J Gastroenterol. 2010;45:560–566. doi: 10.1007/s00535-009-0188-6. [DOI] [PubMed] [Google Scholar]
- 8.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. Gallbladder, AJCC cancer staging manual. 7th ed. New York: Springer; 2010. pp. 211–217. [Google Scholar]
- 9.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 10.Akhurst T, Fong Y. Positron emission tomography in surgical oncology. Adv Surg. 2002;36:309–331. [PubMed] [Google Scholar]
- 11.Anderson CD, Rice MH, Pinson CW, Chapman WC, Chari RS, Delbeke D. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg. 2004;8:90–97. doi: 10.1016/j.gassur.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Corvera CU, Blumgart LH, Akhurst T, DeMatteo RP, D'Angelica M, Fong Y, Jarnagin WR. 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J Am Coll Surg. 2008;206:57–65. doi: 10.1016/j.jamcollsurg.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Kim JY, Kim MH, Lee TY, Hwang CY, Kim JS, Yun SC, Lee SS, Seo DW, Lee SK. Clinical role of 18F-FDG PET-CT in suspected and potentially operable cholangiocarcinoma: a prospective study compared with conventional imaging. Am J Gastroenterol. 2008;103:1145–1151. doi: 10.1111/j.1572-0241.2007.01710.x. [DOI] [PubMed] [Google Scholar]
- 14.Petrowsky H, Wildbrett P, Husarik DB, Hany TF, Tam S, Jochum W, Clavien PA. Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. J Hepatol. 2006;45:43–50. doi: 10.1016/j.jhep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez-Fernández A, Gómez-Río M, Llamas-Elvira JM, Ortega-Lozano S, Ferrón-Orihuela JA, Ramia-Angel JM, Mansilla-Roselló A, Martínez-del-Valle MD, Ramos-Font C. Positron-emission tomography with fluorine-18-fluoro-2-deoxy-D-glucose for gallbladder cancer diagnosis. Am J Surg. 2004;188:171–175. doi: 10.1016/j.amjsurg.2003.12.070. [DOI] [PubMed] [Google Scholar]
- 16.Koh T, Taniguchi H, Yamaguchi A, Kunishima S, Yamagishi H. Differential diagnosis of gallbladder cancer using positron emission tomography with fluorine-18-labeled fluoro-deoxyglucose (FDG-PET) J Surg Oncol. 2003;84:74–81. doi: 10.1002/jso.10295. [DOI] [PubMed] [Google Scholar]
- 17.Kato T, Tsukamoto E, Kuge Y, Katoh C, Nambu T, Nobuta A, Kondo S, Asaka M, Tamaki N. Clinical role of (18)F-FDG PET for initial staging of patients with extrahepatic bile duct cancer. Eur J Nucl Med Mol Imaging. 2002;29:1047–1054. doi: 10.1007/s00259-002-0852-z. [DOI] [PubMed] [Google Scholar]