Fig. 1.
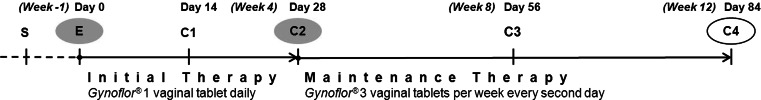
Study design. At screening, eligible patients were included. At Entry visit, an initial tablet of g-Gynoflor® was introduced and a PK study to detect serum estrogens over a 24 h period was performed (Visit E). Patients were checked at 2 weeks for serum estrogen levels, vaginal responses, and side effects (1). At day 28 (Visit C2), 56 (Visit C3), and 84 (Visit C4), the same variables were checked, but on day 28 (Visit C2) another 24 h PK study for serum estrogen dynamics was performed. Between visit E and visit C2 patients applied 1 vaginal tablet daily (Initial therapy phase), whereas after visit 2 Gynoflor® was used every second day (Maintenance phase)
