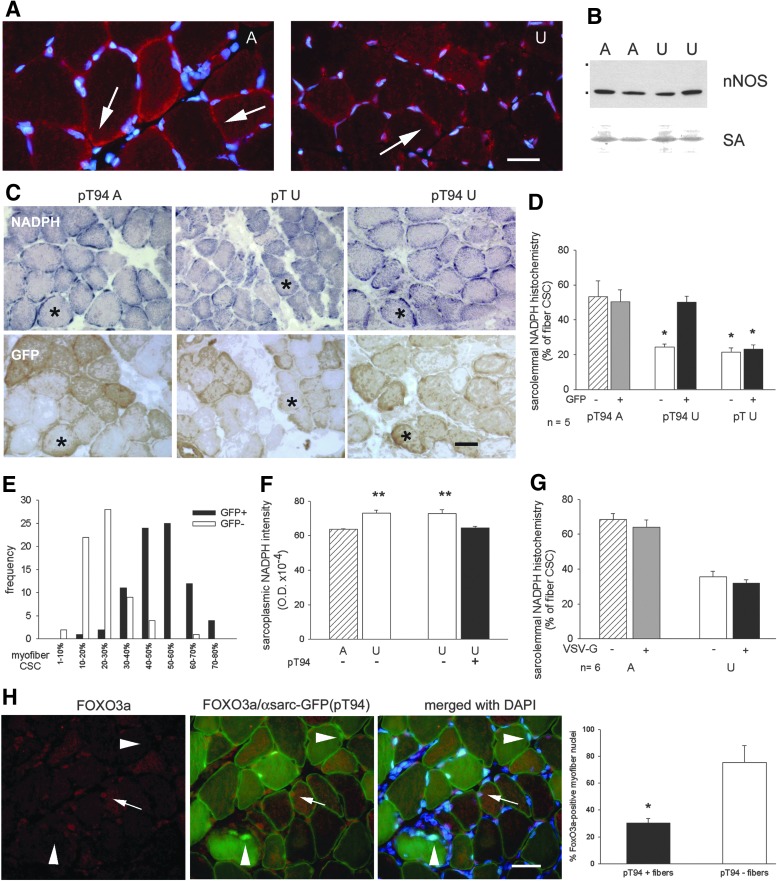
FIG. 6.
Effects of the expression of recombinant or deleted Grp94 on subsarcolemmal nNOS localization of unloaded soleus muscles. Bar: 25 μm. *p<0.01; **p<0.001. (A) Sarcolemmal nNOS immunolabeling in rat soleus muscles. Transverse cryosections of ambulatory (A) and unloaded (U) soleus muscle were reacted with anti-nNOS pAb (red fluorescence) and nuclei counterstained with DAPI (blue fluorescence). Arrows point to reactive, discrete subsarcolemmal regions, which appear reduced by unloading. (B) Western blot for nNOS in rat soleus muscles. Representative blot from a 8% acrylamide denaturing gel loaded with 100 μg of whole muscle homogenates. Dots indicate mobility of molecular weight markers (from the top: 250,000 and 150,000). Reference for sample loading is shown by RP staining for SA. (C) Sarcolemmal nNOS activity, revealed by NADPH-d histochemistry as detailed in “Materials and Methods,” remains detectable in unloaded myofibers after transfection with grp94 cDNA (pT94). Consecutive transverse cryosections were used for NADPH-d enzyme histochemistry (dark blue, upper row) and immunoperoxidase staining with anti-GFP antibodies, to identify transfected myofibers (brown staining, lower row). pT indicates the empty vector. Asterisks indicate representative transfected fibers. Note the presence of discontinuous NADPH-d reactivity at the myofiber sarcolemma of A solei. (D) Recombinant Grp94 expression prevents the unloading-induced loss in sarcolemmal NADPH-d reactivity. Bars and error bars correspond to mean and SE percentage of fiber CSC positive for NADPH-d enzyme histochemistry. Asterisks indicate the presence of significant statistical difference versus both transfected and untransfected myofibers of pT94A muscles and versus GFP+ myofibers of pT94U muscles obtained from rats matched on body weight (p<0.01; within-subject ANOVA test Bonferroni post-hoc test); average n of transfected or untransfected fibers with detectable sarcolemmal NAPDH-d reactivity evaluated in a same muscle: 15 each. n indicates the number of muscles evaluated in each group. (E) Distribution of CSC percentage corresponding to positive sarcolemma for NADPH-d enzyme histochemistry among pT94 transfected fibers (n=79) and untransfected ones (n=66) obtained from 7 U muscles. (F) Recombinant Grp94 expression prevents the unloading-induced increase in intensity of sarcoplasmic NADPH-d enzyme histochemistry. Bars and error bars correspond to mean and SE values of OD. Asterisks indicate the presence of significant statistical difference between untransfected myofibers of A and U muscles (Student's t-test) and between untransfected and pT94-transfected myofibers of U muscles (paired Student's t-test); average n of fibers evaluated for each group: 30 from 3 different muscles. (G) Δ1-570 Grp94 expression does not prevent the loss in sarcolemmal NADPH-d enzyme histochemistry. Bars and error bars correspond to mean and SEM percentage of fiber CSC positive for NADPH-d staining. About 15 transfected (VSV-G+) and untransfected (VSV-G−) fibers with detectable sarcolemmal NAPDH-d activity were evaluated in each muscle. Unloading significantly decreased CSC of both transfected and untransfected fibers compared to A ones evaluated from muscles obtained from rats matched on body weight (Within-subject ANOVA and Bonferroni post-hoc analysis p<0.01). (H) Triple immunofluorescence staining of representative transverse cryosections of pT94-transfected unloaded soleus muscles with anti-FOXO3a antibodies (red fluorescence), anti-α-sarcoglycan antibody (sarcolemmal green fluorescence). and DAPI (blue fluorescence). Sarcoplasmic green fluorescence corresponds to GFP and identifies transfected myofibers. Arrows indicate a representative FOXO3a positive nucleus in an untransfected myofiber (pink fluorescence after merging with DAPI). Arrowheads point to nuclei in transfected myofibers that are unstained for FOXO3a. Histograms represent quantitative evaluation of FOXO3a-positive nuclei in myofibers of unloaded solei in the presence and in the absence of pT94 transfection. Bars and error bars correspond to mean and SE values of the percentage of FOXO3a-positive nuclei evaluated on the total amount of myonuclei detected in transfected and in untrasfected myofibers. Data were collected from six micrographic fields from two muscles for a total number of 400 myofiber nuclei. CSC, cross-sectional circumference; DAPI, 4′,6-diamidino-2-phenylindole; NADPH-d, NAPDH diaphorase; OD, optical density.