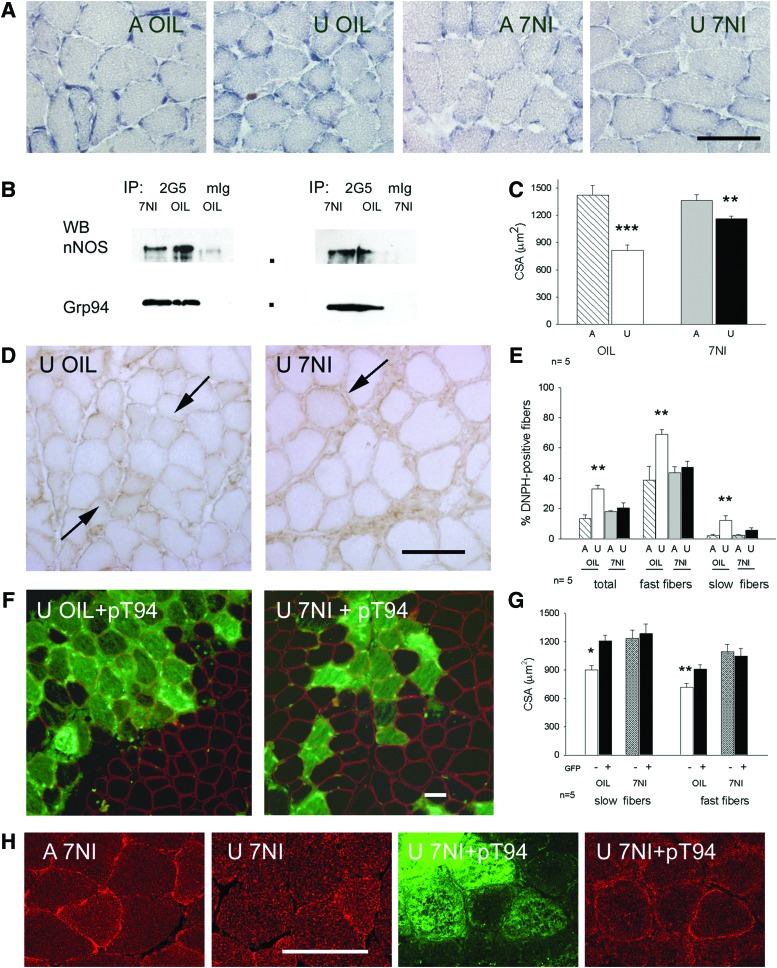
FIG. 7.
Effects of the expression of recombinant Grp94 in unloaded solei after inhibition of nNOS with 7-NI. Dots indicate mobility of molecular weight markers (from the top: 150,000; 100,000 Da). Bar: 50 μm. *p<0.05; **p<0.01, ***p<0.005. (A) Sarcolemmal nNOS activity, revealed by NADPH-d histochemistry, disappeared after treatment with 7-NI. Transverse cryosections of ambulatory (A) and unloaded (U) soleus muscles obtained from rats treated with vehicle (OIL) or with 7-NI were used for NADPH-d histochemistry. Note the presence of positive, discontinuous, NADPH-d activity at the myofiber sarcolemma of A OIL solei. (B) Grp94 immunoprecipitates nNOS in 7-NI solei. Representative western blots of 7.5% acrylamide gels loaded with immunoprecipitates (IP) of protein lysates from ambulatory solei of OIL and 7-NI treated rats, obtained using anti-Grp94 mAb 2G5 or non-immune mIg. Anti-nNOS mAb and anti-Grp94 pAb were used for nitrocellulose staining. (C) 7-NI treatment attenuates CSA decrease of unloaded myofibers. Bars and error bars correspond to mean and SE values of myofiber CSA of A and U solei from OIL and 7-NI treated rats. At least 30 myofibers were evaluated for each muscle, which was considered as a single experimental unit. n indicates the number of muscles evaluated in each group. Asterisks indicate significant difference between U groups and versus the respective A muscle (ANOVA and Bonferroni post-hoc analysis). (D) Presence of myofiber carbonylation in U solei of 7-NI treated rats. Transverse cryosections were exposed to DNPH, as described in “Materials and Methods,” and labeled with anti-DNPH antibodies using indirect immunoperoxidase. Arrows indicate representative carbonylated myofibers. (E) 7-NI treatment decreases the percentage of carbonylated myofibers in unloaded muscles. Bars and error bars correspond to mean and SE values of the percentage of DNPH-positive fibers evaluated on the total myofiber amount of A and U solei of OIL and 7-NI treated rats, and, separatedly, on their respective fast and slow fiber populations. Average n of fibers considered for each muscle: 700. (ANOVA and Bonferroni post-hoc analysis). (F) Visualization of myofibers transfected with Grp94 cDNA (pT94) by means of GFP fluorescence (sarcoplasmic fluorescence) in unloaded muscles of OIL and 7-NI treated rats. Cryosections were counterstained with α-sarcoglycan immunolabeling (sarcolemmal fluorescence) to visualize untransfected fibers for comparison of myofiber CSA. (G) Recombinant Grp94 expression does not affect CSA of unloaded myofibers of 7-NI-treated rats. Bars and error bars correspond to mean and SE values of CSA of pT94 transfected and untransfected slow and fast myofibers of unloaded muscles of OIL and 7-NI-treated rats. At least 20 myofibers (GFP+ and GFP−, each) were evaluated for a same muscle, which was considered as a single experimental unit. CSA values of untransfected fast or slow myofibers of OIL treated rats were significantly lower than those transfected with pT94 in the same muscles and those, either transfected or untransfected, of soleus muscles from 7-NI treated rats matched on body weight (Within-subject ANOVA and Bonferroni post-hoc analysis). (H) Recombinant Grp94 expression affects nNOS immunolocalization in unloaded myofibers of 7-NI-treated rats. Confocal microscopy images after immunofluorescence staining with anti-nNOS pAb (red fluorescence) of A and U solei. Green fluorescence identifies myofibers transfected with Grp94 cDNA (pT94). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars