Figure 1.
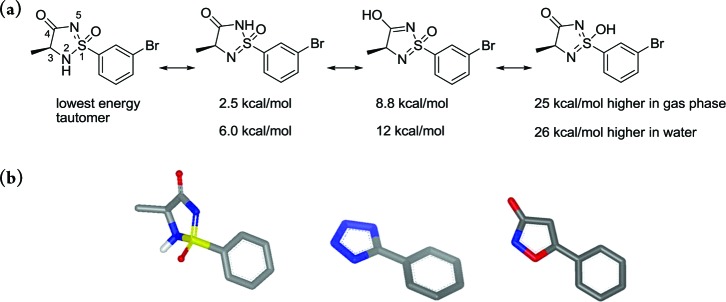
(a) Four different tautomeric forms can be adopted by the cyclic sulfonimidamide ring-system. Calculations show that the tautomer with the acidic proton situated on N2 (left molecule depicted above) has the lowest energy. (b) Calculated structure of the 1S,3S stereoisomer of the cyclic sulfonimidamide depicted in part a and also the corresponding tetrazole and isoxazol-3-one.6
