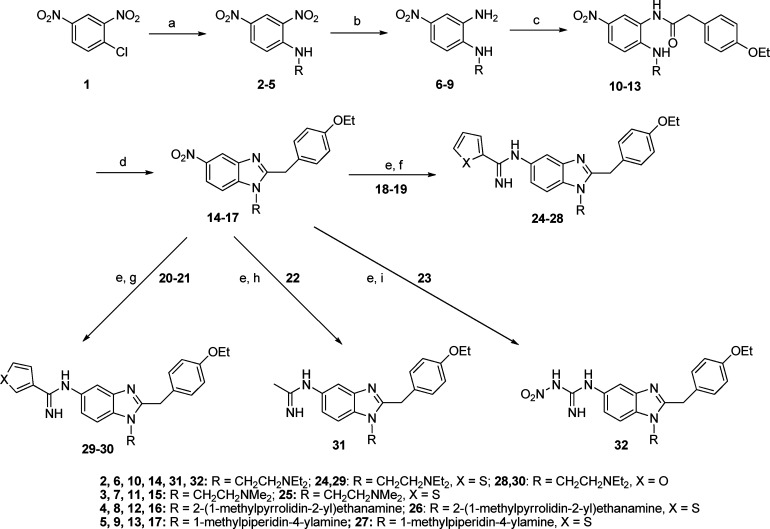
Scheme 1. Synthesis of Trisubstituted Benzimidazole Final Compounds 24–32.
Reagents and conditions: (a) Various diamines (H2NR), EtOH, reflux. (b) Aqueous (NH4)2S, EtOH, H2O, 70 °C. (c) 2-(4-Ethoxyphenyl)acetic acid, EEDQ, CH2Cl2 or THF, 35–60 °C. (d) PCl5, CHCl3, reflux. (e) Pd–C/H2, EtOH, room temperature. (f) Methyl thiophene-2-carbimidothioate·HI (18) or benzyl furan-2-carbimidothioate·HBr (19), EtOH, room temperature. (g) Benzyl thiophene-3-carbimidothioate·HBr (20) or benzyl furan-3-carbimidothioate·HBr (21), EtOH, room temperature. (h) Naphthalen-2-ylmethyl ethanimidothioate·HBr (22), EtOH, room temperature. (i) 1-Methyl-3-nitro-1-nitrosoguanidine (23), EtOH, reflux.