Abstract
The influence of the microenvironment on tumour progression is becoming clearer. In this Review we address the role of an essential signalling pathway, that of transforming growth factor-β, in the regulation of components of the tumour microenvironment and how this contributes to tumour progression.
The transforming growth factor-β (TGFβ) pathway has been established as essential for cancer progression, because of its prominent role in the regulation of cell growth, differentiation and migration. Through the canonical and non-canonical arms of the signalling pathway, TGFβ instigates cellular phenotypical changes that mediate its role as both a tumour suppressor and a tumour promoter. Indeed, the first described phenotypical effect of TGFβ signalling was the induction of a cellular cytostatic programme1 and this provided the first evidence of the TGFβ pathway being tumour suppressive2. However, there was also evidence to the contrary, such as increased tumour progression in carcinomas that overexpressed the TGFβ1 ligand3,4. The initial in vitro evidence for the pro-tumorigenic effects of TGFβ consisted of the induction of a mesenchymal phenotype in epithelial tumour cells (commonly known as an epithelial-to-mesenchymal transition (EMT)) after prolonged exposure to TGFβ5,6.
These early studies into the functional outcome of active TGFβ signalling underlie the difficulties in implementing clinically efficacious treatment regimens that target the TGFβ pathway. The contextual cues that drive the tumour suppressor and tumour promoter roles of TGFβ, as well as the switch between these two phenotypes, are not fully understood. As the understanding of tumour progression has increased, the importance of the tumour microenvironment has been clearly shown. It is interesting to note that TGFβ signalling, both endogenously in human disease and in genetically engineered mouse models of cancer, is associated with the characteristic epithelial changes outlined above, as well as with substantial changes in the tumour stroma7–9; for example, TGFβ1 expression in invasive breast cancer correlates with markers of tumour progression, such as metastasis, extracellular matrix (ECM) deposition and the infiltration of immune cells7. Such findings have laid the groundwork for recent studies that have established TGFβ signalling as an important mediator not only of changes to the epithelial phenotype but also of changes in the stromal environment that are essential for tumour progression. In this Review, we address the functional role of TGFβ signalling in modulating the tumour microenvironment and how these changes affect tumour progression.
The basics of TGFβ signalling
TGFβ1, TGFβ2 and TGFβ3 ligands function as the primary mediators of TGFβ signalling1,10,11 and are secreted as inactive homodimeric polypeptides that can bind to latent TGFβ-binding proteins, which promote extracellular sequestration12. On activation, the ligands bind to the type 2 TGFβ receptor (TGFBR2), which causes recruitment and phosphorylation of TGFBR1 (also known as ALK5), resulting in downstream signalling activation13 (FIG. 1). The strength of this signal depends on which ligand is bound, as the ligands vary in their binding affinity for TGFBR1. This variation in ligand binding promotes differential ligand presentation to TGFBR2 (REF.14). TGFBR3 can augment the initialization of the signalling cascade by promoting differential ligand binding15. The final heterotetrameric form of the active receptors initiates downstream signalling through either SMAD-mediated canonical signalling or SMAD-independent non-canonical signalling13. The canonical signalling pathway involves phosphorylation of the carboxy-terminal serine residue of the internal modulator SMAD proteins, SMAD2 or SMAD3, by the activated receptors16,17. This phosphorylation induces oligomerization of SMAD2 or SMAD3 with SMAD4, which is necessary for nuclear translocation18. Through interactions with a variety of transcription cofactors, the nuclear-localized SMAD complex initiates transcriptional activation or transcriptional repression of several genes. The non-canonical branch of the signalling pathway19 involves activation of the PI3K–AKT, RHOA and MAPK pathways, among others, by the activated heterotetrameric receptors20.
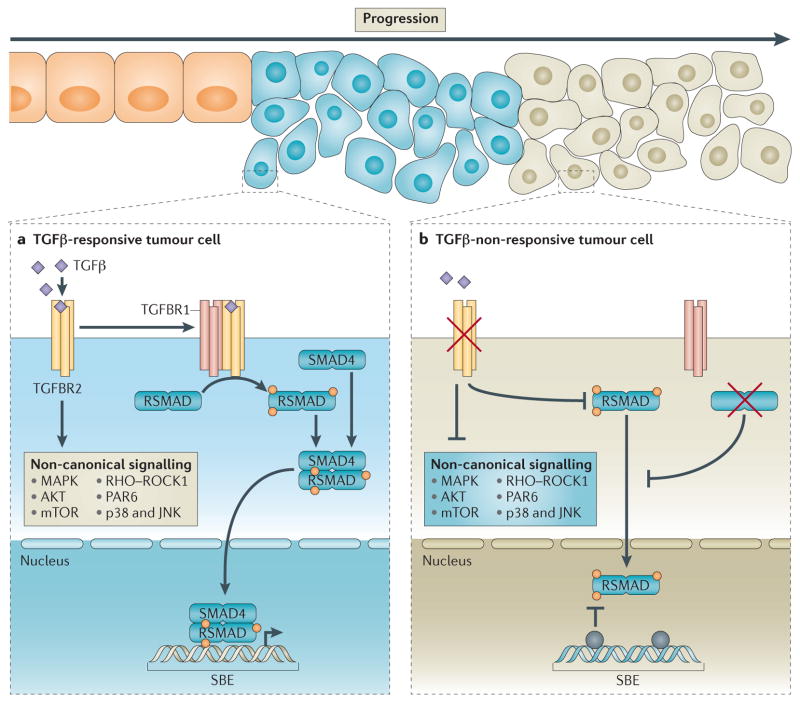
Figure 1. Epithelial TGFβ signalling during tumour progression.
a | Normal transforming growth factor-β (TGFβ) signalling in TGFβ-responsive cells (blue cells) feeds through the type 2 TGFβ receptor (TGFBR2) to activate downstream signalling targets. Canonical signalling is activated through phosphorylation of TGFBR1 to induce nuclear localization and transcriptional activity of SMADs. Non-canonical signalling can occur independently of SMAD proteins and includes activation of RHOA, AKT and MAPK pathways. Early in tumorigenesis, TGFβ functions as a tumour suppressor, partly through the SMAD-dependent induction of cell cycle arrest. b | One hypothesis is that selective pressure leads to the expansion of the population of tumour cells that harbour inactivating mutations in the TGFβ pathway, thus allowing them to overcome the growth-inhibitory effects of active TGFβ signalling. As outlined by Levy et al.48, loss of TGFβ responsiveness (green cells) can occur through loss-of-function mutations, loss of expression or promoter methylation of genes (shown by the grey circles) that encode TGFβ receptors or SMADs. JNK, JUN N-terminal kinase; mTOR, mammalian target of rapamycin; PAR6, partitioning defective 6 homologue; ROCK1, RHO-associated protein kinase 1; RSMAD, receptor SMAD; SBE, SMAD-binding element.
The outcome of these signalling pathways in epithelial cells is either suppression of cell proliferation or induction of cellular migration and invasion. Studies investigating the cytostatic phenotype induced by TGFβ have established numerous pathways through which this is achieved, including repression of MYC and cyclin-dependent kinase 4 (CDK4), as well as the induced expression of CDK inhibitors p21 (also known as CIP1; encoded by CDKN1A) and INK4B (also known as p15)21–23. Furthermore, the SMAD-dependent activation of Krueppel-like factor 10 (also known as TIEG1), death-associated protein kinase 1 (DAPK1) and the pro-apoptotic protein BCL-2-interacting mediator of cell death (BIM; also known as BCL2L11), among others, triggers programmed cell death24. Studies elucidating the tumour suppressor role of TGFβ corroborated evidence that the loss of TGFβ-signalling components was associated with carcinoma progression25,26. The signalling pathway downstream of EMT induction by TGFβ has also been partly mapped. Inhibitor of DNA binding 1 (ID1; a transcriptional regulator) is inhibited by TGFβ. This results in decreased expression of E-cadherin (also known as cadherin 1) and zona occludens 1 (ZO1; also known as TJP1), which are two factors known to help maintain an epithelial phenotype. TGFβ signalling also induces the expression of EMT-associated transcription factors, such as snail family zinc finger 1 (SNAI1), SNAI2, zinc finger E-box binding homeobox 1 (ZEB1), ZEB2, and lymphoid enhancer-binding factor 1 (LEF1), which help to promote the loss of cellular adhesions and cytoskeletal rearrangement27. Non-canonical signalling pathways activated by TGFβ, particularly the RHO–ROCK1 (RHO-associated protein kinase 1) and the AKT pathways, were also shown to be essential in the promotion of the migratory and invasive cellular phenotypes observed after treatment of epithelial cells with TGFβ28. RHO–ROCK1 signalling that is induced by TGFβ is particularly important in single-cell migration. Interestingly, abrogation of TGFβ signalling did not completely stop epithelial cell migration, but rather it switched the cells towards a cohesive migratory phenotype29. Thus, the dichotomous effects of TGFβ signalling are associated with tumour suppression early in tumour development, through initiation of growth arrest, and with tumour promotion in late-stage tumours, through the induction of EMT and of cellular migration and invasion.
However, the effects of epithelial TGFβ signalling on cells that constitute the tumour microenvironment have more recently aided our understanding of the diverse effects that TGFβ has on tumour development and progression. These effects have also started to put in perspective why many studies cite increased metastasis and poor patient prognosis in cancers that lack intact TGFβ signalling.
Disruption of TGFβ signalling
Even without the introduction of oncogenic changes, altered TGFβ-signalling in normal epithelial cells results in phenotypical changes in the stroma; for example, expression of constitutively active TGFBR1 in the mammary epithelium results in mammary glands with greater collagen deposition around the ductal epithelium30. Notably, mammary and pancreatic carcinoma cells that harbour activated TGFBR1 induce a significant increase in angiogenesis following implantation into mice31,32 (FIG. 2). Active TGFβ signalling in mammary tumour cells also promotes tumour progression through the SMAD-independent induction of the expression of matrix metalloproteinases (MMPs), which results in increased angiogenesis and in tumour cell invasion31. One of the more interesting effects of disrupted TGFβ signalling in the malignant epithelium on the tumour microenvironment is related to the alteration of microRNA (miRNA) regulation. miRNAs, which are potent modulators of mRNA, are aberrantly expressed in numerous cancer types and are linked with several pro-tumorigenic and antitumorigenic functions33. TGFβ signalling can inhibit the function of specific miRNAs; for example, TGFβ expression in hepatocellular carcinoma (HCC) cells induces the expression of CC-chemokine ligand 22 (CCL22) through the inhibition of miR-34a expression34. This promotes the recruitment of regulatory T (TReg) cells. TGFβ can also have direct effects on cells that form part of the tumour stroma; for example, in human vascular smooth muscle cells (VSMCs), TGFβ can promote the cleavage and the maturation of pri-mir-21 sequences by inducing the formation of a complex that involves SMAD3 and ATP-dependent RNA helicase DDX5, which is a component of the DROSHA microprocessor complex35. This increases the expression of miR-21, which alters the contractile properties of the VSMCs. TGFβ signalling also increases the expression of miR-29a in human and mouse endothelial cells, which thereby increases the induction of angiogenesis in a non-tumour model36. miR-29a targets phosphatase and tensin homolog (PTEN) mRNA, and it thereby increases the activity of the AKT signalling pathway. TGFβ can also increase the expression of miR-494 in myeloid-derived suppressor cells (MDSCs). This increases the CXC-chemokine receptor (CXCR4)-mediated chemotaxis of MDSCs to the tumour and it reduces the expression of PTEN. The reduced expression of PTEN results in increased AKT activity and in increased expression of pro-tumorigenic liver arginase 1 (ARG1), MMP2, MMP13 and MMP14, which contribute to increased tumour cell invasion and metastasis37.
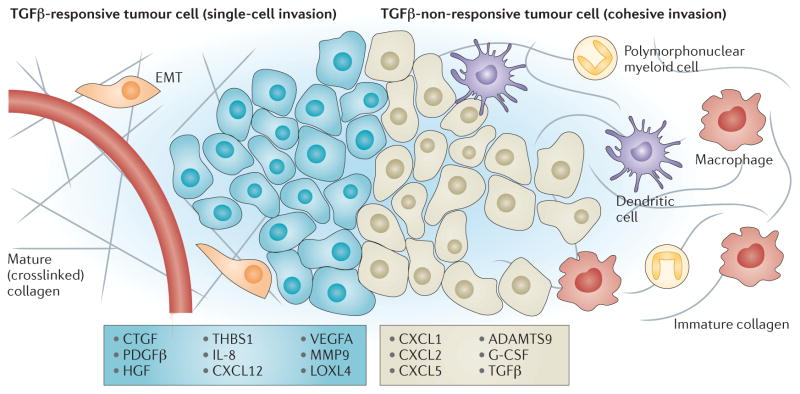
Figure 2. TGFβ signalling in tumour cells determines microenvironmental modification.
Transforming growth factor-β (TGFβ) signalling in tumour cells induces the expression of numerous mediators of extracellular change. Tumours in which cells show increased TGFβ activity are characterized by increased extracellular matrix (ECM) deposition; they show increased secretion of matrix proteins and maturation of these proteins through ECM-modifying enzymes such as lysyl oxidase homologue 4 (LOXL4). In addition, TGFβ signalling in tumour cells drives the induction of endothelial cell recruitment and proliferation, which promote increased angiogenesis. Conversely, TGFβ suppresses the expression of numerous cytokines and chemokines such as the CXC-chemokine ligand 1 (CXCL1) and CXCL5. Loss of TGFβ responsiveness relieves this suppression and results in enhanced immune cell infiltration. These microenvironmental changes promote epithelial cell and stromal cell phenotypical responses, which substantially affect tumour progression. The figure shows the phenotypical changes that specifically result from the epithelial-derived factors listed. ADAMTS9, a disintegrin and metalloproteinase with thrombospondin motifs 9; CTGF, connective tissue growth factor; EMT, epithelial-to-mesenchymal transition; G-CSF, granulocyte colony-stimulating factor; HGF, hepatocyte growth factor; IL-8, interleukin-8; MMP, matrix metalloproteinase; PDGFβ, platelet-derived growth factor-β; THBS1, thrombospondin 1; VEGFA, vascular endothelial growth factor A.
Induction of TGFβ signalling through genetic alterations or through the treatment of carcinoma cells with TGFβ has identified numerous gene targets, many of which are conserved in various cancer types38–41. These experiments have begun to address the functional importance of gene expression induced by TGFβ by correlating these changes with patient data that predict poor patient outcome38,42. Importantly, many of these gene expression targets have well-characterized functions in the modification of tumour microenvironments and they link with phenotypical changes seen on alteration of TGFβ signalling in mouse models of cancer (FIG. 2). TGFβ signalling in epithelial cells induces the expression of numerous ECM genes, including collagen type 1 α1 (COL1A1) and COL4A1, as well as matrix modifying enzymes MMP2, MMP9 and lysyl oxidase homologue 4 (LOXL4). These same studies also provide supporting evidence for the observed pro-angiogenic phenotypes that are induced by TGFβ activation in epithelial cells. Gene expression analysis showed a significant increase in the expression levels of vascular endothelial growth factor A (VEGFA) and thrombospondin 1 (REFS 38–41). Given the EMT and migratory phenotypical changes induced following activation of TGFβ signalling, as well as the epithelial cell TGFβ gene signature associated with tumour recurrence, the identification of stromal changes confirms epithelial cell TGFβ signalling as a pro-tumorigenic signalling pathway. However, such investigations do not distinguish the epithelial and stromal effects of epithelial cell TGFβ signalling on the promotion of tumour cell metastasis, and thus they do not address the specific contribution of each effect to the correlation of TGFβ signalling with poor patient prognosis.
As tumours progress, the growth-inhibitory effects of TGFβ are overcome by the loss of TGFβ pathway elements or of downstream signalling targets10,43 (FIG. 1). Loss of TGFβ responsiveness in tumour cells has substantial effects on tumour progression, not only through altered epithelial cell characteristics but also through gene expression changes that affect the tumour microenvironment. Activated TGFβ signalling has primarily been associated with an increase in metastasis and with poor patient prognosis, because EMT is induced29,44. However, abrogation of TGFβ signalling in carcinoma cells can also result in increased metastasis45. Loss of TGFβ signalling components in both mouse models of cancer and in human cancer has been associated with poor prognosis as a result of increased progression and metastasis25,46–49. Similarly to receptor activation in the normal mammary epithelium, the pancreatic epithelium expressing a dominant-negative TGFBR2 results in increased desmoplasia and angiogenesis in adult mice25. The abrogation or attenuation of TGFβ signalling, either at the receptor or at the SMAD level in mouse models of cancer, has various expected epithelial effects, such as loss of growth inhibition, but it also induces several stromal changes, including activation of stromal fibroblasts, deposition of collagenous ECM, infiltration of a variety of immune cells and increased angiogenesis50. Recent work has shown that the infiltration of MDSCs is increased in tumours in which epithelial TGFβ signalling is abrogated51 (FIG. 2). The increased recruitment of MDSCs is primarily associated with the increased expression of the CXC-chemokine ligand 1 (CXCL1) and CXCL5, the expression of which is normally inhibited by TGFβ. Although this altered chemokine expression pattern is associated with increased myeloid cell infiltration into the tumour microenvironment, these chemokines can also drive the activation of stromal fibroblasts through the induction of connective tissue growth factor (CTGF) expression52. Perhaps the most interesting induced change in gene expression to result from abrogation of TGFβ signalling in epithelial cells is the change in the expression of the TGFβ ligand itself 53,54. Given that the epithelium expressing TGFβ can no longer respond to it, any effects derived from its expression would either be on a separate population of epithelial cells that retain their ability to respond to TGFβ or on cells found in the stromal microenvironment.
The epithelial loss of TGFBR2 results in increased expression of CXCL1, CXCL5 and bone marrow stromal antigen 2 (BST2), and in a reduction in the expression of genes such as CXCL12, platelet-derived growth factor-β (PDGFB) and CTGF55 (FIG. 2). These gene expression changes significantly correlate with a worse outcome in patients with lymph node-positive, oestrogen receptor (ER)-positive luminal A-type breast cancer. These findings introduce an interesting concept that explains many of the counter-intuitive findings regarding impairment of epithelial cell TGFβ signalling that ultimately promotes metastasis. If, as many studies have shown, the stromal microenvironment is a prognostic factor for metastasis, it could override the previously established cell-autonomous signalling events that are associated with tumour cell migration, invasion and metastasis56. Thus, even though tumour cells that lack TGFβ responsiveness no longer gain any epithelial-centric metastatic advantages from TGFβ production, stromal alterations as a result of altered TGFβ expression might continue to promote metastasis. Tumours are heterogeneous and therefore probably contain cells with and without the ability to respond to TGFβ — a concept that is yet to be fully appreciated in terms of its effects on tumour progression.
The effects of TGFβ on stromal cells
Although the effects of increased TGFβ1 expression on epithelial tumour progression have been well established, consideration of the tumour stroma has recently garnered a considerable amount of momentum. Although they give no indication of whether they are causal or merely an effect of progression, specific gene expression changes in the stroma are noted at defined stages of breast cancer progression57. Thus, much like the Vogelgram identified the specific genetic changes that are associated with the stages of colon cancer progression58, we are beginning to appreciate that gene expression changes that correspond with tumour progression also occur in the stroma of tumours. A recent review examined the various effects of stromal components on each hallmark of cancer and highlighted the importance of stromal cells in influencing tumour progression59. However, the mechanisms behind these changes and the pathways that drive specific gene expression changes have yet to be fully elucidated. Given the pleiotropic nature of TGFβ and its established role in regulating the development and the function of numerous stromal cells that have an effect on tumour progression, in this section we discuss components of the tumour microenvironment and examine the role of TGFβ signalling in each.
MSCs
TGFβ has numerous roles in the maintenance and the function of mesenchymal stem cells (MSCs). MSCs have origins in both the bone marrow and a variety of other tissues and have been ascribed numerous functions in normal physiology, including maintaining a homeostatic bone marrow environment and differentiating into various cells that are required for the physiological function of the bone marrow60. In terms of normal physiological function, TGFβ promotes MSC proliferation through induction of the nuclear accumulation of β-catenin, which is mediated by phosphorylation of SMAD3 (REF. 61). In addition, an essential function of MSCs in the bone marrow is the preservation of haematopoietic stem cells in an undifferentiated and inactive state60. This is achieved through the secretion of numerous factors, including TGFβ. Another known function of MSCs is that in various conditions they have the capacity to differentiate into osteoblasts, adipocytes, chondrocytes and fibroblasts62. This differentiation maintains the bone marrow microenvironment, mediating bone turnover and cartilage stability, and facilitating adaptability of tissue homeostasis through the modulation of cell population dynamics. In both normal and malignant conditions, TGFβ mediates the differentiation process, primarily the induction of chondrocyte and fibroblast differentiation and the inhibition of adipocyte differentiation63–65. Although many functions in MSCs are driven by TGFβ, the contextual cues that mediate these differential responses remain unknown. As seen in MSC proliferation, it is probable that the induction of different responses is due to cooperation of the TGFβ pathways with other signalling pathways. TGFβ also modulates MSC function by promoting chemotaxis to sites of tissue damage and to neoplastic lesions63,66.
In tumours, MSCs have been shown to promote tumour progression both on their own and through their ability to differentiate into other cell types67,68. MSCs secrete numerous cytokines that increase tumour cell proliferation, that drive angiogenesis and that affect tumour cell resistance to chemotherapeutic drugs. MSCs in particular can increase tumour metastasis through interactions with carcinoma cells to induce CCL5 expression62,69. Interestingly, this does not seem to be dependent on the ability of CCL5 to function as a chemoattractant for immune cells but rather on its ability to promote cell colonization in the lungs. In addition, as we discuss below, MSCs can inhibit T cell proliferation and activation, which substantially increases their tumour-promoting effects. Another normal physiological function of MSCs is their ability to differentiate into other cells of mesenchymal origin70. Reports indicate that approximately 20% of cancer-associated fibroblasts (CAFs) have origins in the bone marrow, arising from the differentiation of MSCs70. Once differentiated, these fibroblasts promote tumour progression in a TGFβ-dependent manner; indeed, inhibition of TGFβ-mediated MSC differentiation into CAFs through the expression of BAMBI (BMP and activin membrane-bound inhibitor homologue) has been shown to abolish the ability of MSCs to promote tumour progression71.
Fibroblasts
One of the most common features of carcinomas that overexpress TGFβ is a desmoplastic stromal environment7,72–75. Increased ECM deposition corresponds well with the known functions of TGFβ in fibroblast activation and cancer11,50. TGFβ has previously been shown to induce myofibroblast differentiation in fibroblasts, which leads to increased collagen deposition and ECM remodelling76,77. Expression of a constitutively active TGFBR1 in dermal fibroblasts in mice resulted in increased fibrosis and increased expression of known fibroblast-specific TGFβ target genes in chemically induced tumours78. Thus, an abundance of TGFβ in a tumour would probably elicit a similar response. This has been shown to be true as tumours that are characterized as having increased levels of TGFβ are associated with increased fibroblast activation and collagen deposition. In addition, fibroblasts derived from small-cell lung carcinomas show enrichment in TGFβ signalling compared with normal lung fibroblasts, and the TGFβ-enriched gene signature of the carcinoma-derived fibroblasts predicts poor patient outcome79. In fact, it has been shown that the increased TGFβ activation is caused by interactions between stromal fibroblasts and colon carcinoma cells inducing the expression of numerous MMPs and known TGFβ target genes80. As discussed above, numerous studies show that TGFβ-mediated signalling mechanisms are involved in the reciprocal interactions of tumour cells and the stroma. However, only recently have the effects of these interactions on the metastatic potential of tumours been appreciated. Overexpression of TGFβ in a TGFβ-non-responsive colon carcinoma cell line drives increased metastasis of these tumour cells81. In this case, TGFβ expression altered the expression of TGFβ target genes in fibroblasts, including interleukin-11 (IL11), angiopoietin-like 4 (ANGPTL4) and CTGF (TABLE 1). Importantly, the TGFβ-driven gene signature from these fibroblasts was used to predict recurrence in human patients with colon cancer81. As Calon et al.81 comment, such TGFβ-induced gene expression changes have also been ascribed to epithelial carcinoma cells; thus, regardless of the ability of the tumour cells to respond to TGFβ, the pro-tumorigenic effects of the TGFβ-induced gene expression profile are seen in tumours that express high levels of TGFβ.
Table 1.
TGFβ signalling affects central components of fibroblast function
| Factors affected by TGFβ | Expression levels increased | Expression levels decreased |
|---|---|---|
| Chemokines |
|
|
| Growth factors |
|
|
| Matrix deposition |
|
|
| ECM remodelling |
|
|
ADAMTS, a disintegrin and metalloproteinase with thrombospondin motifs; COX2, cyclooxygenase 2; CTGF, connective tissue growth factor; CXCL, CXC-chemokine ligand; EGF, pro-epidermal growth factor; HGF, hepatocyte growth factor; IL, interleukin; IFNγ, interferon-γ; LIF, leukaemia inhibitory factor; LOX, lysly oxidase; MMP, matrix metalloproteinase; iNOS, inducible nitric oxide synthase; TGFβ, transforming growth factor-β; THBS1, thrombospondin 1; TIMP, tissue inhibitor or metelloproteinase; TNF, tumour necrosis factor; VEGF, vascular endothelial growth factor.
Much like epithelial cell TGFβ signalling, work investigating the role of fibroblast TGFβ on tumour progression has showed a contradictory role for the pathway with respect to tumour progression. Although there is a preponderance of evidence supporting a tumour-promoting role for TGFβ in stromal fibroblasts, there is also a considerable amount of work showing a tumour-suppressive role. The foundation of this work lies in the paper from Bhowmick et al.82, which shows that specific deletion of Tgfbr2 in mouse fibroblasts results in spontaneous carcinoma initiation. Further work has shown that fibroblasts that lack TGFβ signalling increase the progression of breast, prostate and squamous cell cancers, as well as melanoma83–85. The major gene expression changes that are associated with the loss of this signalling axis in fibroblasts are in genes that encode cytokines and chemokines. In particular, increased expression of CXCL1, CXCL5, CXCL12 and TGFB1 is observed in fibroblasts that have abrogated TGFβ signalling86 (TABLE 1). This indicates that modulation of the tumour microenvironment through increased infiltration of immune cells is a primary driver of the enhanced tumour progression seen when fibroblasts lose TGFβ responsiveness9,87. Consistent with this idea, recent work shows that a significant increase in inflammation is observed in spontaneous forestomach carcinomas that develop as a result of abrogation of TGFβ signalling in fibroblasts88. Interestingly, this inflammatory response seems to drive tumour formation through the silencing of CDKN1A via epigenetic promoter methylation (BOX 1). Administration of anti-inflammatory drugs significantly delayed tumour onset and increased overall survival — a result that supports immune cell infiltration as a facilitator of tumour development. This work is in agreement with TGFBR2 expression having an important role during the progression of breast cancer from normal to ductal carcinoma in situ (DCIS) and then to invasive ductal carcinoma (IDC). Stromal TGFBR2 expression decreases as these tumours progress towards invasiveness57. Interestingly, immunohistochemical studies in patients with colon cancer have shown a similar trend, but have gone one step further to show that low expression of TGFBR2 in the stroma is an independent predictor of poor patient prognosis87.
Box 1. TGFβ induces epigenetic changes to modulate cellular functions.
Epigenetic modulation of gene expression primarily occurs through modification of histones to promote access to specific stretches of DNA and through DNA methylation of CpG islands in gene promoter regions to modulate transcription factor binding and promoter activity130. The occurrence of altered chromatin structure and of promoter hypermethylation is important in numerous cancer types. In this regard, tumour progression is associated with an increase in histone deacetylase (HDAC) and DNA methyltransferase (DNMT) activity, which drives these changes131,132. Most transforming growth factor-β (TGFβ) silencing in tumours is a direct result of promoter methylation of TGFβ signalling components133–135.
TGFβ and the TGFβ signalling pathway have been linked with histone and promoter modifications136. The hypermethylation of promoter regions of a gene mainly results in the silencing of that gene. This seems to be essential to overcome the tumour-suppressor actions of TGFβ signalling in epithelial cells. Numerous cancer types show hypermethylation of runt-related transcription factor 3 (RUNX3), which results in attenuation of its growth-suppressive functions137. Active TGFβ signalling is required for the maintenance of this methylation, as tumours overexpressing SMAD7 (which inhibits TGFβ signalling), lose methylation of promoters and reverse the effects of gene silencing138. Conversely, it has recently been shown that TGFβ downregulates DNMT expression in fibroblasts139. Fibroblasts have an inverse requirement for promoter methylation with regard to induction of TGFβ target gene expression. DNMT inhibitors enhance the expression of α-smooth muscle actin, as well as collagen type 1, following TGFβ treatment139,140. Thus, in the context of TGFβ signalling, the use of DNMT inhibitors might provide efficacious results with respect to epithelial cells but might ultimately result in increased microenvironmental desmoplasia in these tumours.
The transcriptional activation of SMAD2 target genes requires histone acetylation by p300 before assembly of transcriptional machinery to drive gene expression141. Conversely, induction of a myofibroblast phenotype by TGFβ requires histone deacetylation by HDAC4 (REF. 142). Interestingly, although histone deacetylation is required for TGFβ-mediated fibroblast activation, histone methylation is needed to drive the expression of the TGFβ target genes connective tissue growth factor (CTGF), collagen type 1 α1 (COL1A1) and plasminogen activator inhibitor type 1 (PAI1)143. Such data support the hypothesis that tumour-promoting or -suppressing functions of TGFβ are dependent not only on the microenvironmental context of the tumour but also on the epigenetic histone modifications found in these cells. Although there is a big push for the use of HDAC and DNMT inhibitors in tumour therapy, it is interesting to note that treatment with an HDAC inhibitor might inhibit TGFβ activation in epithelial cells; however, this might increase the response of stromal cells to TGFβ and promote stromal activation.
The innate immune system
The innate immune system is the first line of defence during an immune response. It comprises various phagocytic and antigen-presenting cellular components, including macrophages, neutrophils, natural killer (NK) cells and dendritic cells. TGFβ has a substantial role in regulating the recruitment, activation and function of these cells89. TGFβ functions as an antagonist of major immune functions, decreasing tumour cell recognition and clearing90. One of the most notable functions of TGFβ in this regard is the induction of M2 macrophage polarization91. The expression of inducible nitric oxide synthase (iNOS), which is a driver of the nitric oxide production that suppresses monocyte-mediated cell death, is associated with this polarization92. TGFβ has a similar effect on neutrophils as it has on macrophages93. A recent publication shows that exposure of neutrophils to TGFβ results in a pro-tumorigenic N2 neutrophil expression profile and that inhibition of TGFβ signalling switches this cellular phenotype to an antitumorigenic N1 phenotype94. N2 neutrophils are associated with the expression of pro-tumorigenic factors, such as MMP9, CXCL1 and ARG1.
Rather than inducing a different activation state in NK cells, TGFβ has been identified as a factor that inhibits the functional maturation of this cell population95. Immature NK cells do not respond to foreign antigens, thus exposure of NK cells to TGFβ will impair the recognition and the clearance of tumour cells. The inhibition of maturation also prevents the systemic effects of NK cells, for example, activation of antigen-presenting dendritic cells and inhibition of interferon-γ (IFNγ) secretion, which drives T helper 1 cell (TH1 cell) maturation96,97. However, TGFβ also has antagonistic effects on dendritic cells that inhibit their antitumorigenic properties98. Recent work has shown that impaired TGFβ signalling in dendritic cells significantly increases the ability of these cells to present foreign antigens and to activate the adaptive immune system99. Such actions would promote the killing of tumour cells but, in TGFβ-rich environments where dendritic cells respond to TGFβ, their maturation would be impaired, thus promoting tumour growth and progression. Infiltrating dendritic cells in human lung cancer samples are characterized as immature compared with peritumoural dendritic cells, and this immature state has been shown to promote the differentiation of TReg cells, which increases tumour progression100,101.
The adaptive immune system
Early work investigating the function of TGFβ established that it is essential in the regulation of the adaptive immune system; indeed, systemic loss of TGFβ1 expression results in the development of autoimmune-mediated inflammation in numerous organs102,103. In addition, abrogation of TGFβ signalling specifically in T cells results in a similar autoimmune response104,105. The mechanisms proposed for regulation of adaptive immunity by TGFβ are the SMAD-mediated inhibition of T cell proliferation and the suppression of a TH1 cell phenotype106. Recent work has also shown that TGFβ mediates the immunosuppressive differentiation of T cells89. Treatment of naive T cells with TGFβ induces the expression of the transcription factor forkhead box protein P3 (FOXP3), which drives the phenotypical conversion of a naive T cell to a TReg cell107,108. Interestingly, TReg cell-induced suppression of the adaptive immune response is also mediated through the expression of TGFβ109–111. Addition of IL-6 to the TGFβ treatment of naive T cells induces a completely different cellular phenotype to that of TReg cells112. IL-6 and TGFβ induce TH17 cell differentiation through the suppression of FOXP3 and the activation of the transcription factor retinoic acid receptor-related orphan receptor γt (RORgt)113. Various pro-tumorigenic and antitumorigenic functions of TH17 cells are primarily mediated through the expression of IL-17 in these cells. Interestingly, the function of IL-17 is also context dependent: IL-17 functions as a tumour promoter by inducing angiogenesis and tumour cell proliferation in immune-deficient hosts, but functions as a tumour suppressor in hosts with intact immune systems by enhancing antitumour immune functions. In addition to the secretion of IL-17, the presence of TH17 cells has been associated with increased tumorigenesis that is mediated by neutrophil recruitment and differentiation to an N2 phenotype, and by the induction of IL-6 secretion114.
Despite the numerous pro-tumorigenic functions that are induced by TGFβ stimulation of adaptive immune cells, work using TGFβ signalling-deficient T cells supports an antitumorigenic function for TGFβ signalling in T cells. Thus, as in epithelial cells and fibroblasts, TGFβ signalling in T cells seems to have a contextual dependence that drives its dichotomous functions in tumour progression. Attenuation of TGFβ signalling in CD8+ T cells, through the expression of a dominant-negative TGFBR2, results in uncontrolled cellular proliferation152. Seminal work from the Letterio laboratory115 has shown that genetic deletion of Smad4 in T cells results in the spontaneous development of gastrointestinal tumours. The abundant expression of TH2 cell cytokines has been shown to mediate this phenotype. Just as TGFBR2-deficient fibroblasts are capable of inducing epithelial hyperplasia, so are T cells that are deficient in TGFβ signalling. Counter-intuitively, analysis of SMAD4-deficient T cells shows a significant enrichment of TH17 cells116. Tumours that arise in mice with Smad4-null T cells are characterized by an increased expression of IL-6 and IL-11. This increased expression seems to be sufficient to induce TH17 cell conversion in T cells despite the previously established requirement for TGFβ in this process. However, these studies only examined the canonical TGFβ pathway. The requirement for the non-canonical pathway in the induction of TH17 cells has yet to be addressed.
Although the individual components of both the innate and the adaptive immune system undoubtedly have an important role in tumour progression, it is important to think systemically when considering therapeutic intervention; all cells involved in an immune response interact with and respond to the other components; granulocytes secrete chemokines to promote recruitment of phagocytes and antigen-presenting cells; dendritic cells clear abnormal cells and activate T cells; and so on. Taking a macroscopic view in the context of TGFβ signalling, it can be concluded that TGFβ signalling in the immune system primarily functions as an antagonist that suppresses the recognition and the clearance of tumour cells. It is this feature that has driven current efforts to use anti-TGFβ therapeutic regimens to activate T cell activity and thus to improve current chemotherapeutics.
Conclusions and therapeutic perspectives
Epithelia that lack the ability to respond to TGFβ have various developmental defects, but the loss of TGFβ signalling in selected organs is not, in itself, tumorigenic. However, introduction of oncogenic manipulations causes these cells to be more aggressive and metastatic than their TGFβ-responsive counterparts45,46,49,53. Although many of the pro-tumorigenic effects of TGFβ have been ascribed to the ability of the protein to promote an EMT in epithelial cells, the recently identified effects of this cytokine on the stroma have proved to be an equally important pro-metastatic mechanism. Given the important effects seen in tumour progression following manipulation of TGFβ responsiveness, efficacious intervention in the TGFβ pathway remains a highly sought-after goal for many researchers. As a result of evidence that supports the idea that loss of TGFBR in epithelial cells has a profound effect on the stroma, as well as showing that increased ligand expression by the tumour is associated with poor patient prognosis, the tumour microenvironmental effects of this pathway are increasingly relevant. Rakesh Jain has long been a proponent of the stromal normalization hypothesis, which states that inhibition and reversal of the stromal changes that occur during tumour formation would slow tumour progression and would aid therapeutic intervention117. The use of VEGF inhibitors and relaxin, which is a pregnancy-related hormone shown to induce collagen remodelling, has given credence to this hypothesis; TGFβ inhibitors have recently been added to this list118,119. The use of conventional chemotherapeutics with the specific TGFβ-targeted antibody 1D11 as well as nonspecific inhibitors such as losartan (which is an angiotensin II receptor inhibitor that has been shown to reduce active TGFβ levels in tumours), significantly increases the delivery and the efficacy of chemotherapeutic regimens. The use of these inhibitors in mouse models promoted vessel stability, decreased collagen deposition and significantly improved the efficacy of doxorubicin and doxil treatments120.
Numerous studies have shown that systemically intervening in the TGFβ pathway during cancer progression elicits antitumorigenic effects on epithelial cell phenotypes and through microenvironmental changes (TABLE 2). Several Phase I and Phase II clinical trials of TGFβ-neutralizing antibodies and of small-molecule inhibitors to both ligand and receptor have so far been shown to be safe and efficacious; many trials are still accruing patients121–124. For a complete and comprehensive examination of therapeutic treatments targeting the TGFβ pathway in malignant and non-malignant disease progression, see REF. 125.
Table 2.
Identified microenvironmental effects of TGFβ inhibitors in preclinical tumour models
| Drug name | Cancers tested | Microenvironmental effects | Refs |
|---|---|---|---|
| TGFβ ligand trap | |||
| P144 | Melanoma | Increased dendritic cell, natural killer cell and T cell activity | 144 |
| 1D11 | Lung, breast and glioblastoma |
|
119,145, 146 |
| TGFβ antisense | |||
| AP12009 | Pancreatic | Increased immune cell-mediated cytotoxicity | 123 |
| Receptor inhibitors | |||
| GW788388 | Oesophageal squamous cell carcinoma |
|
147 |
| LY2109761 | Hepatocellular carcinoma and glioblastoma |
|
148,149 |
| LY364947 | Glioblastoma | Increased vascular permeability | 146 |
| LY3022859 | Breast, pancreas and colon |
|
126 |
| SB431542 | Breast | Increased dendritic cell maturation | 98 |
| SD-208 | Breast cancer bone metastasis and glioma |
|
127 |
| SX-007 | Glioma | Increased presence of CD3+ T cells | 150 |
| SM-16 | Lung |
|
151 |
MDSC, myeloid-derived suppressor cells; TGFβ, transforming growth factor-β; TReg, regulatory T.
Interestingly, studies show that the effects of TGFβ inhibition are efficacious even in the treatment of tumours that lack the central mediator of canonical TGFβ signalling, SMAD4 (REF. 126). Such data highlight the importance not only of the microenvironment but also of microenvironmental TGFβ signalling on tumour progression. TGFβ-inhibitory treatment regimens tested in numerous mouse models of different cancer types, as well as in early-stage clinical trials, have shown benefits; increased activity of antitumoural adaptive immune cells is thought to be responsible127,128. However, inhibition of the TGFβ signalling pathway in specific components of the microenvironment has once again introduced controversial findings into the field of TGFβ research: suppression of TGFβ signalling in fibroblasts, in an effort to reduce the desmoplastic response in these cells, promoted tumour progression through the augmentation of inflammatory cell infiltration81,88. Despite this, clinical trials involving TGFβ inhibition, either through genetic abrogation of TGFβ sensitivity (such as by the adoptive transfer of T cells expressing dominantnegative forms of the TGFBR) or through the use of inhibitory antibodies, have progressed (ClinicalTrials.gov identifiers: NCT00368082, NCT01582269 and NCT00844064). Although modest and mixed results are consistently obtained from these trails, this is probably due to varying stromal compositions and to the pleiotropic effects of TGFβ.
Studies that elucidate the function of TGFβ signalling in specific cellular components of the stroma and that establish an efficacious means of specifically targeting the pathways in these cells are imperative in the resolution of the contextual dependence of TGFβ on the tumour microenvironment. Indeed, it is probable that, as with epithelial cell TGFβ signalling, there is a contextual dependence that controls the tumour-promoting or tumour-suppressing actions of TGFβ in the tumour microenvironment. As cellular components and their interactions drive this microenvironmental context, future studies will need to address not only signalling but also the stromal milieu in the observed response. Without a thorough understanding of the contextual dependence of TGFβ signalling in the stroma it is probable that the implementation of systemic therapeutics that target the TGFβ pathway will yield inconclusive results because of tumour heterogeneity. Thus, novel methods of delivery that facilitate specific targeting to a cellular type or evasion of pathway activation are being investigated, including the adoptive transfer of T cells that have an abrogated TGFβ response, thus circumventing the antitumorigenic response to TGFβ129. This highly specific manipulation should provide beneficial effects on tumour progression, without introducing controversial results by affecting TGFβ signalling in other stromal and epithelial cells.
Key points.
During the early stages of tumorigenesis, transforming growth factor-β (TGFβ) functions as a tumour suppressor. However, as tumours progress, tumour cells may lose their growth-inhibitory response to TGFβ and may instead respond by initiating epithelial-to-mesenchymal transition and by increasing cell migration.
Experimental data support the idea that both loss of and gain of TGFβ signalling is pro-tumorigenic, as mouse models show that the overexpression of TGFβ and the abrogation of signalling results in increased tumour cell metastasis. It is beginning to be appreciated that the effects of TGFβ signalling in the tumour epithelium extend beyond tumour cell-autonomous mechanisms into the tumour microenvironment.
Nearly every cell in the tumour microenvironment responds to TGFβ in a unique way, and these diverse biological responses have a variety of effects on tumour progression.
Given the pleotropic nature of TGFβ signalling, the numerous downstream signalling events of TGFβ offer new and intriguing targets for therapeutic intervention.
Successful therapeutic targeting of TGFβ itself remains a highly desirable goal and the considerable advances in understanding TGFβ signalling, not only in the tumour epithelium but also in the tumour microenvironment, bring the realization of this goal closer.
Acknowledgments
This work is supported by US National Institutes of Health (NIH) Grants CA085492 and CA102162. The authors would also like to thank all of the members of the Moses Laboratory for their thoughtful discussions. In addition, the authors would like to apologize to any investigators whose work could not be included owing to space limitations.
Glossary
- Epithelial-to-mesenchymal transition
The process by which epithelial cells lose their epithelial characteristics, such as cellular polarity and cell–cell junctions, in favour of mesenchymal characteristics, which include high cellular motility
- Cohesive migratory phenotype
Contrary to single-cell invasion, this is a form of invasion by which cells maintain their cell– cell contacts and by which cells migrate as a unit
- MicroRNA
(miRNA) 20–30 nucleotide long non-coding RNA that modulates gene expression by binding to complementary sequences in the 3′ untranslated region of target genes, which induces repression of gene translation or mRNA degradation
- Myeloid-derived suppressor cells
(MDSCs) A heterogeneous population of cells that are defined by their myeloid origin, immature state and ability to potently suppress T cell responses
- Chemotaxis
The function of a cell responding to a chemokine gradient to promote directional migration towards sites of tissue injury or into tumour microenvironments
- Dominant-negative
A defective protein product that effectively inhibits the function of its wild-type counterpart by retaining essential interaction capabilities without having a desired effector function
- Desmoplasia
The accumulation of extracellular matrix proteins, usually through the enhanced activation of stromal fibroblasts
- Vogelgram
A model for colorectal cancer development in which specific genetic changes are acquired, which mark progression of the disease from benign to malignant
- Myofibroblast
A generalized term for an activated fibroblast. In tumour biology, these cells function to deposit and to remodel the extracellular matrix as well as to alter tumour progression through paracrine protein signalling
- T helper 1 cell
(TH1 cell) A primary effector cell of the adaptive immune system, which is classically defined as antitumorigenic
- 1D11
An antibody which is specifically directed towards the three transforming growth factor-β ligands and used to sequester these ligands, thus inhibiting TGFβ signalling
- Losartan
An angiotensin II receptor antagonist that has been shown to have the off-target effects of downregulating expression of type I transforming growth factor- β receptor (Tgfbr1) and Tgfbr2
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Siegel PM, Massague J. Cytostatic and apoptotic actions of TGFβ in homeostasis and cancer. Nature Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 2.Massague J. G1 cell-cycle control and cancer. Nature. 2004;432:298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 3.Gorsch SM, Memoli VA, Stukel TA, Gold LI, Arrick BA. Immunohistochemical staining for transforming growth factor β 1 associates with disease progression in human breast cancer. Cancer Res. 1992;52:6949–6952. [PubMed] [Google Scholar]
- 4.Hasegawa Y, et al. Transforming growth factor-β1 level correlates with angiogenesis, tumor progression, and prognosis in patients with nonsmall cell lung carcinoma. Cancer. 2001;91:964–971. [PubMed] [Google Scholar]
- 5.Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-β induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127:2021–2036. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caulin C, Scholl FG, Frontelo P, Gamallo C, Quintanilla M. Chronic exposure of cultured transformed mouse epidermal cells to transforming growth factor-β 1 induces an epithelial-mesenchymal transdifferentiation and a spindle tumoral phenotype. Cell Growth Differ. 1995;6:1027–1035. [PubMed] [Google Scholar]
- 7.Walker RA, Dearing SJ, Gallacher B. Relationship of transforming growth factor β 1 to extracellular matrix and stromal infiltrates in invasive breast carcinoma. Br J Cancer. 1994;69:1160–1165. doi: 10.1038/bjc.1994.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wikstrom P, Stattin P, Franck-Lissbrant I, Damber JE, Bergh A. Transforming growth factor β1 is associated with angiogenesis, metastasis, and poor clinical outcome in prostate cancer. Prostate. 1998;37:19–29. doi: 10.1002/(sici)1097-0045(19980915)37:1<19::aid-pros4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Hazelbag S, Gorter A, Kenter GG, van den Broek L, Fleuren G. Transforming growth factor-β1 induces tumor stroma and reduces tumor infiltrate in cervical cancer. Hum Pathol. 2002;33:1193–1199. doi: 10.1053/hupa.2002.130109. [DOI] [PubMed] [Google Scholar]
- 10.Bierie B, Moses HL. TGF-β and cancer. Cytokine Growth Factor Rev. 2006;17:29–40. doi: 10.1016/j.cytogfr.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Massague J. TGFβ signalling in context. Nature Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rifkin DB. Latent transforming growth factor-β (TGF-β) binding proteins: orchestrators of TGF-β availability. J Biol Chem. 2005;280:7409–7412. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- 13.Shi Y, Massague J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 14.Stenvers KL, et al. Heart and liver defects and reduced transforming growth factor β2 sensitivity in transforming growth factor β type III receptor-deficient embryos. Mol Cell Biol. 2003;23:4371–4385. doi: 10.1128/MCB.23.12.4371-4385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moustakas A, et al. The transforming growth factor β receptors types I, II, and III form hetero-oligomeric complexes in the presence of ligand. J Biol Chem. 1993;268:22215–22218. [PubMed] [Google Scholar]
- 16.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 17.Feng XH, Derynck R. Specificity and versatility in TGF-β signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 18.Schmierer B, Hill CS. Kinetic analysis of Smad nucleocytoplasmic shuttling reveals a mechanism for transforming growth factor β-dependent nuclear accumulation of Smads. Mol Cell Biol. 2005;25:9845–9858. doi: 10.1128/MCB.25.22.9845-9858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moustakas A, Heldin CH. Non-Smad TGF-β signals. J Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 20.Massague J, Gomis RR. The logic of TGFβ signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 21.Ewen ME, Oliver CJ, Sluss HK, Miller SJ, Peeper DS. p53-dependent repression of CDK4 translation in TGF-β-induced G1 cell-cycle arrest. Genes Dev. 1995;9:204–217. doi: 10.1101/gad.9.2.204. [DOI] [PubMed] [Google Scholar]
- 22.Polyak K, et al. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 23.Hannon GJ, Beach D. p15INK4B is a potential effector of TGF-β-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 24.Pardali K, Moustakas A. Actions of TGF-β as tumor suppressor and pro-metastatic factor in human cancer. Biochim Biophys Acta. 2007;1775:21–62. doi: 10.1016/j.bbcan.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Bottinger EP, et al. Expression of a dominant-negative mutant TGF-β type II receptor in transgenic mice reveals essential roles for TGF-β in regulation of growth and differentiation in the exocrine pancreas. EMBO J. 1997;16:2621–2633. doi: 10.1093/emboj/16.10.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amendt C, Schirmacher P, Weber H, Blessing M. Expression of a dominant negative type II TGF-β receptor in mouse skin results in an increase in carcinoma incidence and an acceleration of carcinoma development. Oncogene. 1998;17:25–34. doi: 10.1038/sj.onc.1202161. [DOI] [PubMed] [Google Scholar]
- 27.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Dumont N, Bakin AV, Arteaga CL. Autocrine transforming growth factor-β signaling mediates Smad-independent motility in human cancer cells. J Biol Chem. 2003;278:3275–3285. doi: 10.1074/jbc.M204623200. [DOI] [PubMed] [Google Scholar]
- 29.Giampieri S, et al. Localized and reversible TGFβ signalling switches breast cancer cells from cohesive to single cell motility. Nature Cell Biol. 2009;11:1287–1296. doi: 10.1038/ncb1973. This paper shows the identification of TGFβ signalling as a mediator of single-cell invasion and of vascular metastasis, with the cells that are unable to respond to TGFβ showing a cohesive migratory phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muraoka-Cook RS, et al. Activated type I TGFβ receptor kinase enhances the survival of mammary epithelial cells and accelerates tumor progression. Oncogene. 2006;25:3408–3423. doi: 10.1038/sj.onc.1208964. [DOI] [PubMed] [Google Scholar]
- 31.Safina A, Vandette E, Bakin AV. ALK5 promotes tumor angiogenesis by upregulating matrix metalloproteinase-9 in tumor cells. Oncogene. 2007;26:2407–2422. doi: 10.1038/sj.onc.1210046. [DOI] [PubMed] [Google Scholar]
- 32.Schniewind B, et al. Dissecting the role of TGF-β type I receptor/ALK5 in pancreatic ductal adenocarcinoma: Smad activation is crucial for both the tumor suppressive and prometastatic function. Oncogene. 2007;26:4850–4862. doi: 10.1038/sj.onc.1210272. [DOI] [PubMed] [Google Scholar]
- 33.Esquela-Kerscher A, Slack FJ. Oncomirs — microRNAs with a role in cancer. Nature Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 34.Yang P, et al. TGF-β-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell. 2012;22:291–303. doi: 10.1016/j.ccr.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. This is the first established link between SMAD activity and the maturation of pri-miRNA through DROSHA, specifically following BMP treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Wang Y, Ma Y, Lan Y, Yang X. Transforming growth factor β-regulated microRNA-29a promotes angiogenesis through targeting the phosphatase and tensin homolog in endothelium. J Biol Chem. 2013;288:10418–10426. doi: 10.1074/jbc.M112.444463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, et al. MicroRNA-494 is required for the accumulation and functions of tumor-expanded myeloid-derived suppressor cells via targeting of PTEN. J Immunol. 2012;188:5500–5510. doi: 10.4049/jimmunol.1103505. [DOI] [PubMed] [Google Scholar]
- 38.Wang SE, et al. Transforming growth factor β engages TACE and ErbB3 to activate phosphatidylinositol-3 kinase/Akt in ErbB2-overexpressing breast cancer and desensitizes cells to trastuzumab. Mol Cell Biol. 2008;28:5605–5620. doi: 10.1128/MCB.00787-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sartor MA, et al. ConceptGen: a gene set enrichment and gene set relation mapping tool. Bioinformatics. 2010;26:456–463. doi: 10.1093/bioinformatics/btp683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hills CE, Willars GB, Brunskill NJ. Proinsulin C-peptide antagonizes the profibrotic effects of TGF-β1 via up-regulation of retinoic acid and HGF-related signaling pathways. Mol Endocrinol. 2010;24:822–831. doi: 10.1210/me.2009-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maupin KA, et al. Glycogene expression alterations associated with pancreatic cancer epithelial– mesenchymal transition in complementary model systems. PLoS ONE. 2010;5:e13002. doi: 10.1371/journal.pone.0013002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coulouarn C, Factor VM, Thorgeirsson SS. Transforming growth factor-β gene expression signature in mouse hepatocytes predicts clinical outcome in human cancer. Hepatology. 2008;47:2059–2067. doi: 10.1002/hep.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Massague J. TGFβ in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mima K, et al. Epithelial–mesenchymal transition expression profiles as a prognostic factor for disease-free survival in hepatocellular carcinoma: clinical significance of transforming growth factor-β signaling. Oncol Lett. 2013;5:149–154. doi: 10.3892/ol.2012.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Forrester E, et al. Effect of conditional knockout of the type II TGF-β receptor gene in mammary epithelia on mammary gland development and polyomavirus middle T antigen induced tumor formation and metastasis. Cancer Res. 2005;65:2296–2302. doi: 10.1158/0008-5472.CAN-04-3272. [DOI] [PubMed] [Google Scholar]
- 46.Lu SL, et al. Loss of transforming growth factor-β type II receptor promotes metastatic head-and-neck squamous cell carcinoma. Genes Dev. 2006;20:1331–1342. doi: 10.1101/gad.1413306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paiva CE, et al. Absence of TGF-βRII predicts bone and lung metastasis and is associated with poor prognosis in stage III breast tumors. Cancer Biomark. 2012;11:209–217. doi: 10.3233/CBM-2012-00281. This paper is a histochemical study of TGFβ signalling in breast cancer and shows a correlation between decreased signalling in the tumour epithelium and increased tumour metastasis to the lungs and the bone. This article also indicates that low TGFBR2 levels indicate poor disease-free and overall survival. [DOI] [PubMed] [Google Scholar]
- 48.Levy L, Hill CS. Alterations in components of the TGF-β superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev. 2006;17:41–58. doi: 10.1016/j.cytogfr.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 49.Malkoski SP, et al. Loss of transforming growth factor β type II receptor increases aggressive tumor behavior and reduces survival in lung adenocarcinoma and squamous cell carcinoma. Clin Cancer Res. 2012;18:2173–2183. doi: 10.1158/1078-0432.CCR-11-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bierie B, Moses HL. Tumour microenvironment: TGFβ: the molecular Jekyll and Hyde of cancer. Nature Rev Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 51.Yang L, et al. Abrogation of TGF β signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ijichi H, et al. Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J Clin Invest. 2011;121:4106–4117. doi: 10.1172/JCI42754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin S, et al. Attenuation of TGF-β signaling suppresses premature senescence in a p21-dependent manner and promotes oncogenic Ras-mediated metastatic transformation in human mammary epithelial cells. Mol Biol Cell. 2012;23:1569–1581. doi: 10.1091/mbc.E11-10-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gewin L, et al. TGF-β receptor deletion in the renal collecting system exacerbates fibrosis. J Am Soc Nephrol. 2010;21:1334–1343. doi: 10.1681/ASN.2010020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bierie B, et al. Abrogation of TGF-β signaling enhances chemokine production and correlates with prognosis in human breast cancer. J Clin Invest. 2009;119:1571–1582. doi: 10.1172/JCI37480. This paper is the first to establish of a poor clinical outcome related to the genetic changes, particularly the altered chemokine expression, that occur following ablation of TGFβ signalling in the tumour epithelium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Finak G, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nature Med. 2008;14:518–527. doi: 10.1038/nm1764. This is the first identification of a link between stromal gene expression changes and the outcome of patients with breast cancer. [DOI] [PubMed] [Google Scholar]
- 57.Knudsen ES, et al. Progression of ductal carcinoma in situ to invasive breast cancer is associated with gene expression programs of EMT and myoepithelia. Breast Cancer Res Treat. 2012;133:1009–1024. doi: 10.1007/s10549-011-1894-3. [DOI] [PubMed] [Google Scholar]
- 58.Vogelstein B, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 59.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 60.Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nature Rev Mol Cell Biol. 2011;12:126–131. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watabe T, Miyazono K. Roles of TGF-β family signaling in stem cell renewal and differentiation. Cell Res. 2009;19:103–115. doi: 10.1038/cr.2008.323. [DOI] [PubMed] [Google Scholar]
- 62.Cuiffo BG, Karnoub AE. Mesenchymal stem cells in tumor development: emerging roles and concepts. Cell Adh Migr. 2012;6:220–230. doi: 10.4161/cam.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang Y, et al. TGF-β1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nature Med. 2009;15:757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao L, Hantash BM. TGF-β1 regulates differentiation of bone marrow mesenchymal stem cells. Vitam Horm. 2011;87:127–141. doi: 10.1016/B978-0-12-386015-6.00042-1. [DOI] [PubMed] [Google Scholar]
- 65.Kurpinski K, et al. Transforming growth factor-β and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells. 2010;28:734–742. doi: 10.1002/stem.319. [DOI] [PubMed] [Google Scholar]
- 66.Wan M, et al. Injury-activated transforming growth factor β controls mobilization of mesenchymal stem cells for tissue remodeling. Stem Cells. 2012;30:2498–2511. doi: 10.1002/stem.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jung Y, et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nature Commun. 2013;4:1795. doi: 10.1038/ncomms2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quante M, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–272. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karnoub AE, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 70.Spaeth EL, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS ONE. 2009;4:e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shangguan L, et al. Inhibition of TGF-β/Smad signaling by BAMBI blocks differentiation of human mesenchymal stem cells to carcinoma-associated fibroblasts and abolishes their protumor effects. Stem Cells. 2012;30:2810–2819. doi: 10.1002/stem.1251. [DOI] [PubMed] [Google Scholar]
- 72.Verona EV, et al. Transforming growth factor-β signaling in prostate stromal cells supports prostate carcinoma growth by up-regulating stromal genes related to tissue remodeling. Cancer Res. 2007;67:5737–5746. doi: 10.1158/0008-5472.CAN-07-0444. [DOI] [PubMed] [Google Scholar]
- 73.Border WA, Noble NA. Transforming growth factor β in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 74.Leask A, Abraham DJ. TGF-β signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 75.Boyd NF, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 76.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-β 1 induces αalpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-β1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sonnylal S, et al. Postnatal induction of transforming growth factor β signaling in fibroblasts of mice recapitulates clinical, histologic, and biochemical features of scleroderma. Arthritis Rheum. 2007;56:334–344. doi: 10.1002/art.22328. [DOI] [PubMed] [Google Scholar]
- 79.Navab R, et al. Prognostic gene-expression signature of carcinoma-associated fibroblasts in non-small cell lung cancer. Proc Natl Acad Sci USA. 2011;108:7160–7165. doi: 10.1073/pnas.1014506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hawinkels LJ, et al. Interaction with colon cancer cells hyperactivates TGF-β signaling in cancer-associated fibroblasts. Oncogene. 2012 doi: 10.1038/onc.2012.536. http://dx.doi.org/10.1038/onc.2012.536. [DOI] [PubMed]
- 81.Calon A, et al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22:571–584. doi: 10.1016/j.ccr.2012.08.013. This paper establishes TGFβ-responsive stromal signalling, especially from fibroblasts, as a driver of tumour progression and metastasis. This stromal programme could be blocked with the addition of TGFβ inhibitors to slow tumour growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhowmick NA, et al. TGF-β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. This is the first report showing that genetic mutations in stromal fibroblast can result in the development of carcinoma formation from the adjacent epithelium. [DOI] [PubMed] [Google Scholar]
- 83.Cheng N, et al. Loss of TGF-β type II receptor in fibroblasts promotes mammary carcinoma growth and invasion through upregulation of TGF-alpha-, MSPand HGF-mediated signaling networks. Oncogene. 2005;24:5053–5068. doi: 10.1038/sj.onc.1208685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Franco OE, et al. Altered TGF-β signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res. 2011;71:1272–1281. doi: 10.1158/0008-5472.CAN-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meng W, et al. Downregulation of TGF-β receptor types II and III in oral squamous cell carcinoma and oral carcinoma-associated fibroblasts. BMC Cancer. 2011;11:88. doi: 10.1186/1471-2407-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu BJ, et al. Quantitative analysis of the secretome of TGF-β signaling-deficient mammary fibroblasts. Proteomics. 2010;10:2458–2470. doi: 10.1002/pmic.200900701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bacman D, et al. TGF-β receptor 2 downregulation in tumour-associated stroma worsens prognosis and high-grade tumours show more tumour-associated macrophages and lower TGF-β1 expression in colon carcinoma: a retrospective study. BMC Cancer. 2007;7:156. doi: 10.1186/1471-2407-7-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Achyut BR, et al. Inflammation-mediated genetic and epigenetic alterations drive cancer development in the neighboring epithelium upon stromal abrogation of TGF-β signaling. PLoS Genet. 2013;9:e1003251. doi: 10.1371/journal.pgen.1003251. This is an interesting paper showing that inflammatory infiltrates mediate the pro-tumorigenic functions of fibroblasts that lack TGFβ signalling, particularly through the induction of epigenetic silencing of p15 and p16 in epithelial cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFβ. Nature Rev Immunol. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gigante M, Gesualdo L, Ranieri E. TGF-β: a master switch in tumor immunity. Curr Pharm Des. 2012;18:4126–4134. doi: 10.2174/138161212802430378. [DOI] [PubMed] [Google Scholar]
- 91.Gong D, et al. TGFβ signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. 2012;13:31. doi: 10.1186/1471-2172-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nature Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33:949–955. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- 94.Fridlender ZG, et al. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. This is the first paper to establish an N2 neutrophil phenotype and to show that this phenotype is driven by TGFβ signalling. These neutrophils promote tumour progression through the inhibition of antitumorigenic CD8+ T cell activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marcoe JP, et al. TGF-β is responsible for NK cell immaturity during ontogeny and increased susceptibility to infection during mouse infancy. Nature Immunol. 2012;13:843–850. doi: 10.1038/ni.2388. This paper identifies TGFβ as a factor that inhibits NK cell maturation and shows that loss of TGFβ signalling is a mark of stage F NK cell development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leavy O. Maturation and function of NK cells. Nature Rev Immunol. 2012;12:150. doi: 10.1038/nri3172. [DOI] [PubMed] [Google Scholar]
- 97.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-β controls T helper type 1 cell development through regulation of natural killer cell interferon-γ. Nature Immunol. 2005;6:600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 98.Tanaka H, et al. Transforming growth factor β signaling inhibitor, SB-431542, induces maturation of dendritic cells and enhances antitumor activity. Oncol Rep. 2010;24:1637–1643. doi: 10.3892/or_00001028. [DOI] [PubMed] [Google Scholar]
- 99.Novitskiy SV, et al. Deletion of TGF-β signaling in myeloid cells enhances their anti-tumorigenic properties. J Leukoc Biol. 2012;92:641–651. doi: 10.1189/jlb.1211639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Perrot I, et al. Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. J Immunol. 2007;178:2763–2769. doi: 10.4049/jimmunol.178.5.2763. [DOI] [PubMed] [Google Scholar]
- 101.Roncarolo MG, Levings MK, Traversari C. Differentiation of T regulatory cells by immature dendritic cells. J Exp Med. 2001;193:F5–9. doi: 10.1084/jem.193.2.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shull MM, et al. Targeted disruption of the mouse transforming growth factor-β 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kulkarni AB, et al. Transforming growth factor β 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-β receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 105.Bommireddy R, et al. Self-antigen recognition by TGF β1-deficient T cells causes their activation and systemic inflammation. Lab Invest. 2006;86:1008–1019. doi: 10.1038/labinvest.3700460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li MO, Flavell RA. TGF-β: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen W, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tone Y, et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nature Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 109.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 110.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nature Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.von Boehmer H, Daniel C. Therapeutic opportunities for manipulating TReg cells in autoimmunity and cancer. Nature Rev Drug Discov. 2013;12:51–63. doi: 10.1038/nrd3683. [DOI] [PubMed] [Google Scholar]
- 112.Mangan PR, et al. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. This paper identifies TGFβ as a primary factor in the induced differentiation of TH17 cell commitment in a subpopulation of T cells — a process that is independent of TReg cells. [DOI] [PubMed] [Google Scholar]
- 113.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 114.Whiteside TL. What are regulatory T cells (Treg) regulating in cancer and why? Semin Cancer Biol. 2012;22:327–334. doi: 10.1016/j.semcancer.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim BG, et al. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature. 2006;441:1015–1019. doi: 10.1038/nature04846. This seminal paper identifies of TGFβ superfamily signalling in T cells as an inhibitor of tumour progression due to the suppression of the expression of TH1 cytokines. [DOI] [PubMed] [Google Scholar]
- 116.Ghoreschi K, et al. Generation of pathogenic Th17 cells in the absence of TGF-β signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol. 2013;31:2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huang Y, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci USA. 2012;109:17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu J, et al. TGF-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc Natl Acad Sci USA. 2012;109:16618–16623. doi: 10.1073/pnas.1117610109. This is an interesting paper showing that TGFβ inhibition- normalized breast tumour stroma enhances chemotherapeutic efficacy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci USA. 2011;108:2909–2914. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mead AL, Wong TT, Cordeiro MF, Anderson IK, Khaw PT. Evaluation of anti-TGF-β2 antibody as a new postoperative anti-scarring agent in glaucoma surgery. Invest Ophthalmol Vis Sci. 2003;44:3394–3401. doi: 10.1167/iovs.02-0978. [DOI] [PubMed] [Google Scholar]
- 122.Bogdahn U, et al. Targeted therapy for high-grade glioma with the TGF-β2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol. 2011;13:132–142. doi: 10.1093/neuonc/noq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schlingensiepen KH, et al. Transforming growth factor-β 2 gene silencing with trabedersen in pancreatic cancer. Cancer Sci. 2011;102:1193–1200. doi: 10.1111/j.1349-7006.2011.01917.x. [DOI] [PubMed] [Google Scholar]
- 124.Roldan Urgoiti GB, Singh AD, Easaw JC. Extended adjuvant temozolomide for treatment of newly diagnosed glioblastoma multiforme. J Neurooncol. 2012;108:173–177. doi: 10.1007/s11060-012-0826-3. [DOI] [PubMed] [Google Scholar]
- 125.Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nature Rev Drug Discov. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhong Z, et al. Anti-transforming growth factor β receptor II antibody has therapeutic efficacy against primary tumor growth and metastasis through multieffects on cancer, stroma, and immune cells. Clin Cancer Res. 2010;16:1191–1205. doi: 10.1158/1078-0432.CCR-09-1634. This paper shows that anti-TGFβ therapeutics slow tumour progression by modulating the composition of immune cell infiltrates in favour of an antitumorigenic phenotype. [DOI] [PubMed] [Google Scholar]
- 127.Uhl M, et al. SD-208, a novel transforming growth factor β receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res. 2004;64:7954–7961. doi: 10.1158/0008-5472.CAN-04-1013. [DOI] [PubMed] [Google Scholar]
- 128.Kim S, et al. Systemic blockade of transforming growth factor-β signaling augments the efficacy of immunogene therapy. Cancer Res. 2008;68:10247–10256. doi: 10.1158/0008-5472.CAN-08-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Foster AE, et al. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-β receptor. J Immunother. 2008;31:500–505. doi: 10.1097/CJI.0b013e318177092b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Azad N, Zahnow CA, Rudin CM, Baylin SB. The future of epigenetic therapy in solid tumours-lessons from the past. Nature Rev Clin Oncol. 2013;10:256–266. doi: 10.1038/nrclinonc.2013.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nature Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 132.Zhang Q, et al. TGF-β regulates DNA methyltransferase expression in prostate cancer, correlates with aggressive capabilities, and predicts disease recurrence. PLoS ONE. 2011;6:e25168. doi: 10.1371/journal.pone.0025168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ammanamanchi S, Brattain MG. Restoration of transforming growth factor-β signaling through receptor RI induction by histone deacetylase activity inhibition in breast cancer cells. J Biol Chem. 2004;279:32620–32625. doi: 10.1074/jbc.M402691200. [DOI] [PubMed] [Google Scholar]
- 134.Kang SH, et al. Transcriptional repression of the transforming growth factor-β type I receptor gene by DNA methylation results in the development of TGF-β resistance in human gastric cancer. Oncogene. 1999;18:7280–7286. doi: 10.1038/sj.onc.1203146. [DOI] [PubMed] [Google Scholar]
- 135.Osada H, et al. Heterogeneous transforming growth factor (TGF)-β unresponsiveness and loss of TGF-β receptor type II expression caused by histone deacetylation in lung cancer cell lines. Cancer Res. 2001;61:8331–8339. [PubMed] [Google Scholar]
- 136.Khin SS, et al. Epigenetic alteration by DNA promoter hypermethylation of genes related to transforming growth factor-β (TGF-B) signaling in cancer. Cancers. 2011;3:982–993. doi: 10.3390/cancers3010982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yeh KT, et al. Aberrant TGFβ/SMAD4 signaling contributes to epigenetic silencing of a putative tumor suppressor, RunX1T1 in ovarian cancer. Epigenetics. 2011;6:727–739. doi: 10.4161/epi.6.6.15856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Papageorgis P, et al. Smad signaling is required to maintain epigenetic silencing during breast cancer progression. Cancer Res. 2010;70:968–978. doi: 10.1158/0008-5472.CAN-09-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pan X, Chen Z, Huang R, Yao Y, Ma G. Transforming growth factor β1 induces the expression of collagen type I by DNA methylation in cardiac fibroblasts. PLoS ONE. 2013;8:e60335. doi: 10.1371/journal.pone.0060335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hu B, Gharaee-Kermani M, Wu Z, Phan SH. Epigenetic regulation of myofibroblast differentiation by DNA methylation. Am J Pathol. 2010;177:21–28. doi: 10.2353/ajpath.2010.090999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ross S, et al. Smads orchestrate specific histone modifications and chromatin remodeling to activate transcription. EMBO J. 2006;25:4490–4502. doi: 10.1038/sj.emboj.7601332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sun G, et al. Epigenetic histone methylation modulates fibrotic gene expression. J Am Soc Nephrol. 2010;21:2069–2080. doi: 10.1681/ASN.2010060633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Glenisson W, Castronovo V, Waltregny D. Histone deacetylase 4 is required for TGFβ1-induced myofibroblastic differentiation. Biochim Biophys Acta. 2007;1773:1572–1582. doi: 10.1016/j.bbamcr.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 144.Diaz-Valdes N, et al. Induction of monocyte chemoattractant protein-1 and interleukin-10 by TGFβ1 in melanoma enhances tumor infiltration and immunosuppression. Cancer Res. 2011;71:812–821. doi: 10.1158/0008-5472.CAN-10-2698. [DOI] [PubMed] [Google Scholar]
- 145.Terabe M, et al. Synergistic enhancement of CD8+ T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-β monoclonal antibody. Clin Cancer Res. 2009;15:6560–6569. doi: 10.1158/1078-0432.CCR-09-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hardee ME, et al. Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-β. Cancer Res. 2012;72:4119–4129. doi: 10.1158/0008-5472.CAN-12-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Noma K, et al. The essential role of fibroblasts in esophageal squamous cell carcinoma-induced angiogenesis. Gastroenterology. 2008;134:1981–1993. doi: 10.1053/j.gastro.2008.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mazzocca A, Fransvea E, Lavezzari G, Antonaci S, Giannelli G. Inhibition of transforming growth factor β receptor I kinase blocks hepatocellular carcinoma growth through neo-angiogenesis regulation. Hepatology. 2009;50:1140–1151. doi: 10.1002/hep.23118. [DOI] [PubMed] [Google Scholar]
- 149.Zhang M, et al. Trimodal glioblastoma treatment consisting of concurrent radiotherapy, temozolomide, and the novel TGF-β receptor I kinase inhibitor LY2109761. Neoplasia. 2011;13:537–549. doi: 10.1593/neo.11258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tran TT, et al. Inhibiting TGF-β signaling restores immune surveillance in the SMA-560 glioma model. Neuro Oncol. 2007;9:259–270. doi: 10.1215/15228517-2007-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Garrison K, et al. The small molecule TGF-β signaling inhibitor SM16 synergizes with agonistic OX40 antibody to suppress established mammary tumors and reduce spontaneous metastasis. Cancer Immunol Immunother. 2012;61:511–521. doi: 10.1007/s00262-011-1119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lucas PJ, Kim SJ, Melby SJ, Gress RE. Disruption of T. cell homeostasis in mice expressing a T cell-specific dominant negative transforming growth factor β II receptor. J Exp Med. 2000;191:1187–1196. doi: 10.1084/jem.191.7.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]