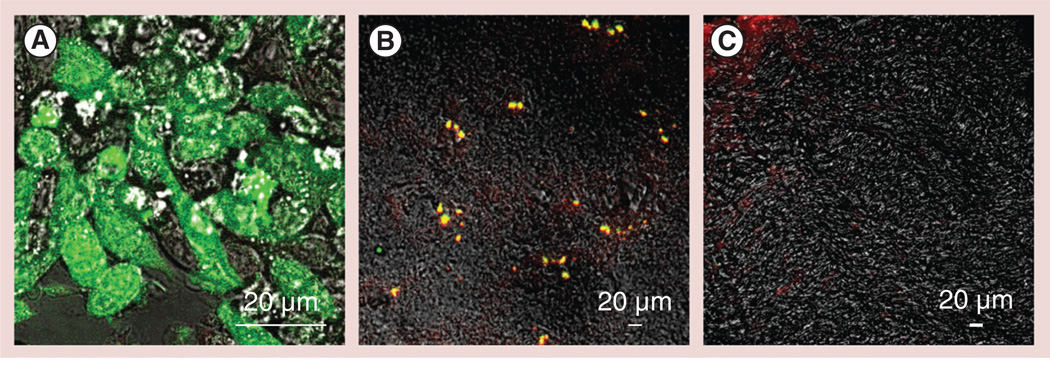
Figure 8. Recruitment of green fluorescent protein-expressing RAW 264.7 macrophages to the substantia nigra pars compacta in 6-hydroxydopamine-intoxicated mice.
(A)Macrophages were transduced with green fluorescent protein (GFP) firefly luciferase virus and selected against katamycin. BALB/c mice were intracranially injected with 6-hydroxydopamine into the substantia nigra pars compacta, as described in the ‘Materials & Methods’ section. A total of 21 days later, the animals were injected into the intrajugular vein with GFP-expressing RAW 264.7 macrophages (green; 5 × 106 cells/ mouse in 100 µl). A total of 24 h later, the mice were sacrificed, and perfused with phosphate-buffered saline and 4% paraformaldehyde. (B) The brains were frozen, sectioned with a cryostat (10 µm thick) and examined by confocal microscopy (60× magnification). (C) Healthy mice without brain inflammation (with phosphate-buffered saline intracranial injections) were used as a control group. Slides were stained with primary antibodies to CD11b, a marker for macrophages, and secondary fluorescently labeled anti-mouse-IgG-atto 647N (red). Colocalization of GFP-expressing macrophages and CD11b antibodies, manifested in yellow staining, confirmed the presence of significant amounts of the genetically modified cells in the intoxicated brain. (C) No fluorescence in the healthy brain was found, indicating that systemically administered RAW 264.7 macrophages did not cross the blood–brain barrier in the absence of brain inflammation.