Figure 1. Biosynthesis of pro-resolving lipid mediators from EPA and DHA.
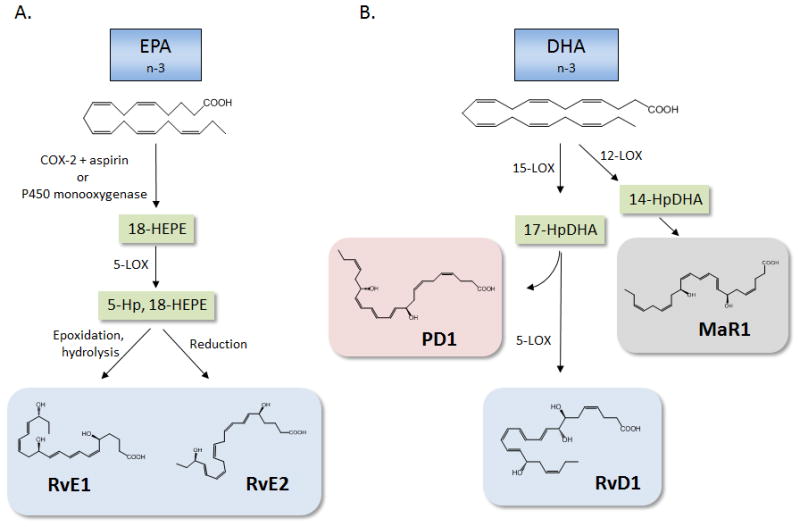
(A) EPA serves as the substrate precursor for the E-series resolvins. In the presence of aspirin, acetylated cyclooxygenase (COX)-2 utilizes EPA as a substrate and produces 18-hydroxy eicosapentaenoic acid (18-HEPE). This intermediate, which can also be generated by a P450 route, can serve as a substrate for 5-lipoxygenase (LOX) to give rise to 5-hydroperoxy (Hp)-18-HEPE. Epoxidation and enzymatic hydrolysis generates RvE1, while 5-Hp, 18-HEPE can also be directly reduced to generate RvE2. (B). DHA is converted to 17-HpDHA by 15-LOX, which, through the formation of an epoxide intermediate, can form protectin D1 (PD1). Conversely, 17-HpDHA can be further converted by 5-LOX to generate resolvin D1 (RvD1). In addition to 15-LOX, DHA can also serve as a substrate for 12-LOX, giving rise to 14-HpDHA, which through enzymatic epoxidation and hydrolysis, gives rise to maresin 1 (MaR1).
