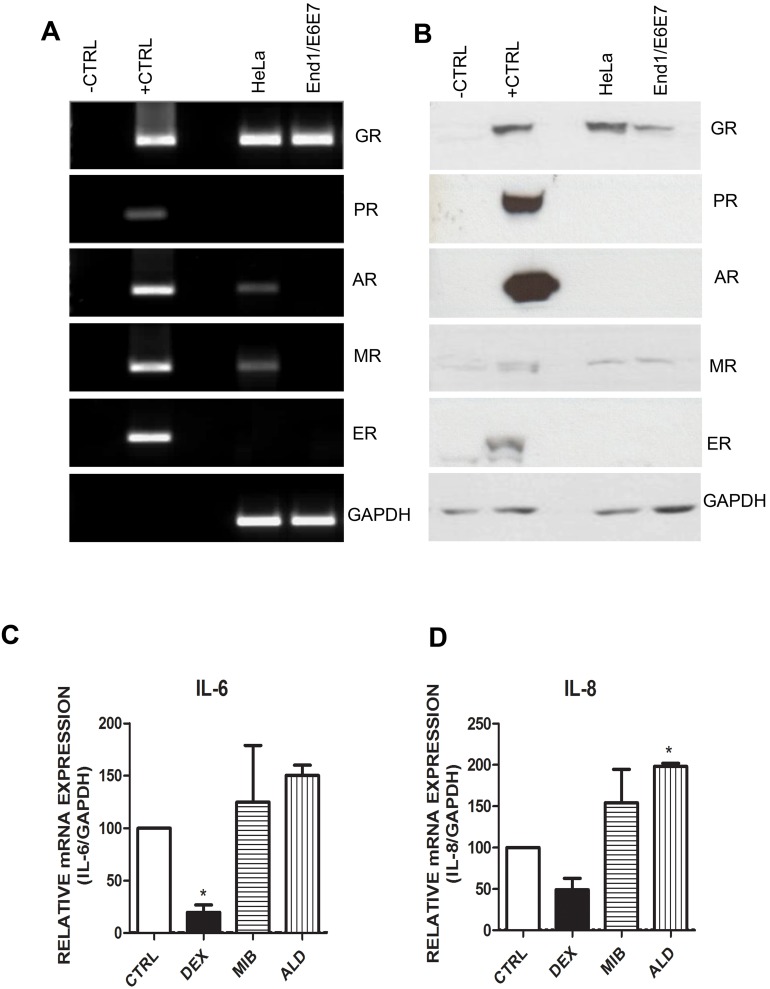
Figure 4. End1/E6E7 and HeLa cells only express detectable GR protein.
(A and B) (A) HeLa and End1/E6E7 cells were harvested, total RNA was isolated and reverse-transcribed. Steroid receptor (SR) gene expression was measured by real time qRT-PCR. SR expression vectors (pcDNA3-hGR, pMT-PR-B, pSV-hAR, pRS-hMR and pSG5-hER) served as positive controls (+CTRL) for the GR, PR-B, MR and ER, respectively. COS-1 cells transiently transfected with pcDNA3 (empty vector) served as negative control (−CTRL). (B) Whole cell lysates were prepared from the HeLa and End1/E6E7 cell lines. Equal volumes of lysate were analysed by Western blotting with antibodies against specific SRs and GAPDH as loading control. (C and D) SR agonist screen indicates that in the cervical cells the GR, but not the MR or AR repress IL-6 and IL-8 in the presence of receptor-specific agonist. HeLa cells were treated with 100 nM DEX, 100 nM mibolerone (MIB), 10 nM aldosterone (ALD) or vehicle (ethanol) (CTRL) for 4 hrs. Total RNA was isolated and reverse-transcribed. Relative (C) IL-6 and (D) IL-8 gene expression was measured by real-time qRT-PCR and normalised to GAPDH mRNA expression. In addition, relative gene expressions were normalized to basal activity (CTRL) in order to obtain fold expression. The primers and antibody used to investigate PR levels are capable of detecting both PR-A and PR-B isoforms, however the positive protein control shown is specific for PR-B isoform only. Graphs represent pooled results of at least three independent experiments and are plotted as mean ± SEM. Statistical analysis was carried out using GraphPad Prism software (version 5) using a one-way ANOVA with Dunnett post-test. Statistical significance is denoted by * to indicate P<0.001.