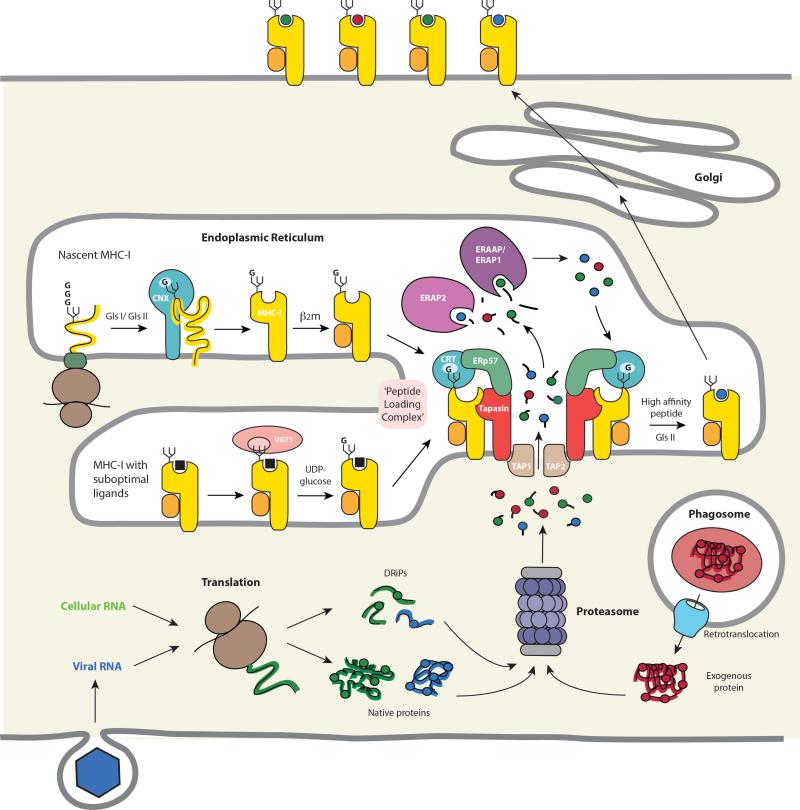
Figure 3.
MHC-I biosynthesis and antigenic peptide binding in the ER. Trimming of the N-linked glycan by glucosidases I and II (GlsI/ GlsII) to a single terminal glucose residue (“G”) permits the interaction of the MHC-I heavy chain with lectin-like chaperones at several stages during folding and assembly. The initial folding events involve the chaperone calnexin (CNX) and allow subsequent assembly with β2m. The empty heterodimer, which is inherently unstable, is then recruited by calreticulin (CRT) via the monoglucosylated N-linked glycan to the PLC. The association of MHC-I/β2m heterodimers with the PLC both stabilizes the empty MHC-I molecule and maintains the binding groove in a conformation that favors high affinity peptide loading. These functions are mediated by direct interactions between the MHC-I heavy chain and tapasin and are supported by coordinating interactions with CRT and ERp57 in the PLC. MHC-I molecules with suboptimal peptides are substrates for UGT1 which reglucosylates the heavy chain glycan, allowing re-entry of the MHC-I into the PLC and exchange for high affinity peptides. Peptides translocated into the ER by TAP originate primarily from the proteasomal degradation of endogenous proteins or DRiPs. These proteins may arise from the translation of either self or foreign (i.e. viral) RNA or, in the case of cross-presentation, by translocation into the cytosol from endosomes or phagosomes. Many of the peptides that are delivered into the ER are longer than the 8-10 residues preferred by MHC-I molecules and undergo trimming by ER aminopeptidases known as ERAAP/ERAP1 and ERAP2. Finally, high affinity peptides bind preferentially to MHC-I molecules in the PLC by a tapasin-mediated editing process, MHC-I-peptide complexes are released and then transit to the cell surface for T cell recognition by CD8+ T cells.