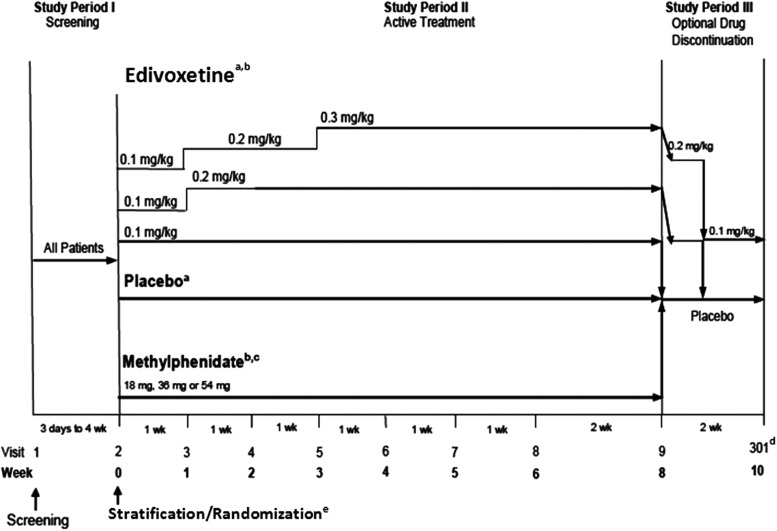
FIG. 1.
Study design. aIncludes patients with prior stimulant treatment and patients with no prior stimulant treatment. bTitration to target dose was based on patient's weight (methylphenidate dose=18 mg [18.0–23.9 kg], 36 mg [24.0–41.9 kg], 54 mg [42.0–≤75 kg]). cIncludes only patients with no prior stimulant treatment. dPatients unable to tolerate the assigned dose during Study Period II were discontinued from the study and returned for a safety follow-up visit at visit 301 (end-point for Study Period III). ePatients were first stratified based on previous stimulant treatment history, then randomized; stimulant-naïve patients were randomized at a ratio of 1:1:1:1:1 to placebo, extended-release methylphenidate, or one of three fixed-dose arms of edivoxetine (targeted doses of 0.1, 0.2, or 0.3 mg/kg); stimulant-prior patients were randomized at a ratio of 1:1:1:1 to placebo or one of three fixed-dose arms of edivoxetine. wk=week.