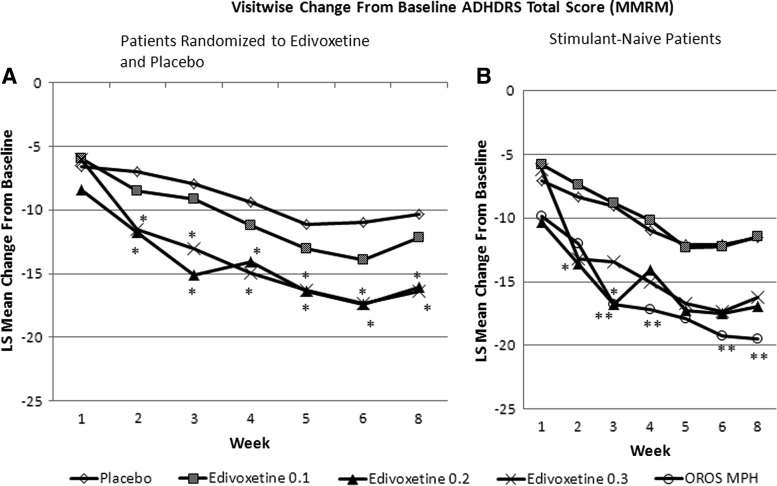
FIG. 3.
Primary efficacy measure in the efficacy analyses data set: Baseline-to-endpoint change in ADHD-RS-IV-Parent:Inv total score according to per-protocol analysis of patients with previous stimulant exposure (A: left panel) and stimulant-naive patients (B: right panel). *p<0.05 edivoxetine vs. placebo; **p<0.05 methylphenidate (MPH) vs. placebo. 0.1=0.1 mg/kg/day; 0.2=0.2 mg/kg/day; 0.3=0.3 mg/kg/day; ADHDRS-IV-Parent:Inv=Attention-Deficit/Hyperactivity Disorder Rating Scale-IV-Parent Version: Investigator Administered and Scored; LS=least squares; MMRM=mixed model repeated measures; OROS MPH=osmotic-release oral system methylphenidate.