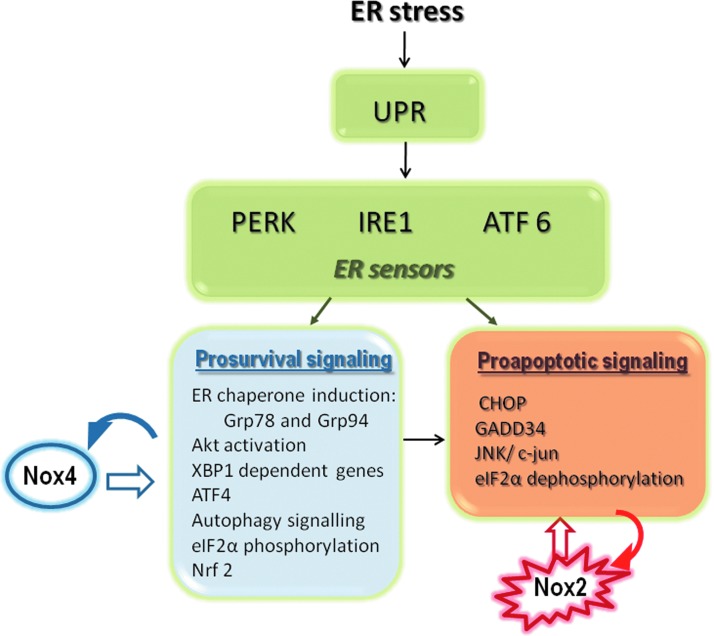
FIG. 1.
Organization of the unfolded protein response (UPR) signaling and interplay with Nox NADPH oxidases. ER stress triggers UPR signaling from three main arms based on distinct ER transmembrane sensors: the endonuclease/kinase IRE1, the kinase PERK, and transcription factor ATF6. Each one triggers signals emerging from the cytosolic surface of ER, which integrate pathways for decreasing global protein synthesis load, improving protein folding, adjusting metabolism and amino acid sufficiency, and inducing misfolded protein degradation. Such responses can be classified as either prosurvival or proapoptotic, although both types are triggered simultaneously. Among several UPR-responsive homeostatic genes, the chaperones Grp78 (BiP), Grp94, and calreticulin are regulators, as well as operational markers, of the UPR. The most specific apoptosis pathway is the transcription factor CHOP (GADD 153). Nox4 is induced early during the UPR and is upstream to several signaling events that may induce autophagy, survival, or eventually apoptosis. Nox2 is induced through a CHOP/CAMKII pathway to mediate apoptosis. CAMKII, calcium/calmodulin-dependent protein kinase II; CHOP, CCAT/enhancer binding protein homologous protein; ER, endoplasmic reticulum; Grp, glucose-regulated protein; JNK, c-Jun N-terminal kinases; NADPH, reduced nicotinamide adenine dinucleotide phosphate. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars