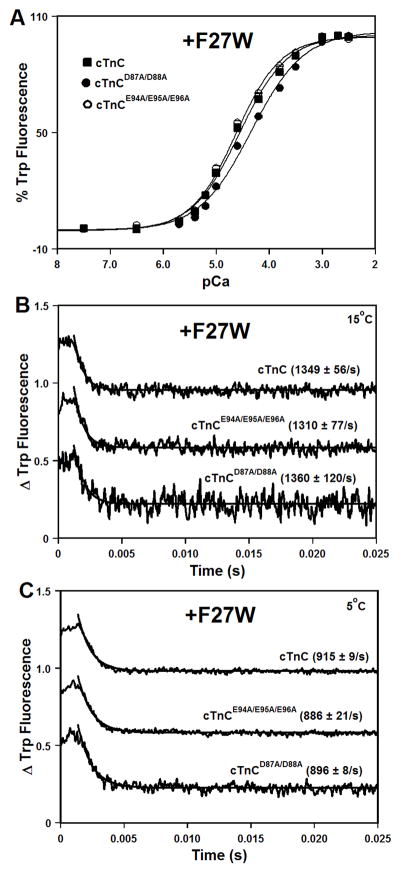
Figure 2. Effect of the central helix mutations on the Ca2+ binding properties of isolated cTnC.
Panel A shows increases in Trp fluorescence, which occur as Ca2+ binds to the N-domains of cTnC (■), cTnCD87A/D88A (●), or cTnCE94A/E95A/E96A (○) at 15°C. The cTnC proteins contained F27W substitution. The Trp fluorescence was excited at 285 nm and monitored at 345 nm. Panels B and C show the time course of decreases in Trp fluorescence as Ca2+ was removed by excess EGTA from the N-domains of isolated cTnC, cTnCD87A/D88A, or cTnCE94A/E95A/E96A. Panel B: measured at 15°C. Panel C: measured at 5°C. The data traces have been normalized and staggered for clarity. Each trace is an average of at least five traces fit with a single exponential equation. The Trp fluorescence was excited at 285 nm and monitored through a WG320 filter.