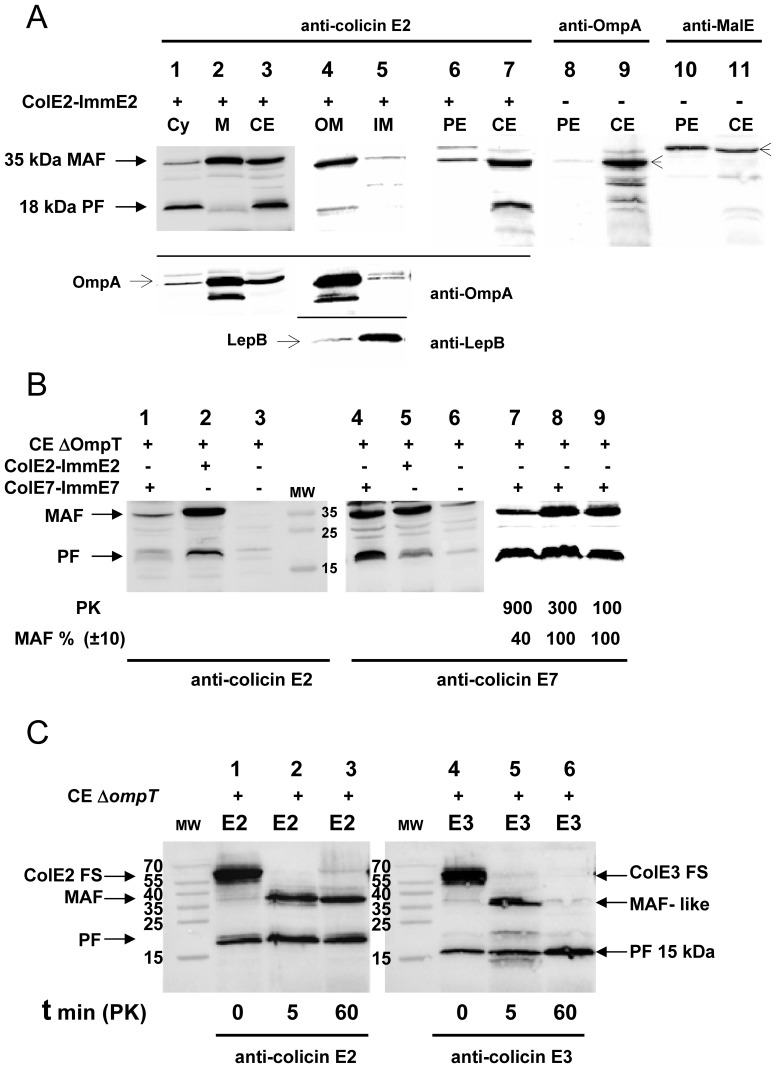
Figure 1. Detection and analysis of the in vivo cleaved forms of DNase colicins E2 and E7.
(A) The proteins of the crude cell extracts, (lanes 3, 7, 9, 11, CE), total membrane (lane 2, M), outer membrane (lane 4, OM), inner membrane (lane 5, IM) and the S100 cytoplasmic (lanes 1, Cy) fractions, isolated from colicin E2-ImmE2 treated (lanes 1–7, +) or from non treated (lanes 8–11, -) cells of an ompT-inactivated strain AD202, were separated on 15% SDS-PAGE and analysed by Western blotting with anti-colicin E2 antiserum (lanes 1–7, upper part of the panel). The membrane attached form (MAF) and the processed form (PF) with MW of about 35 and 18 kDa, respectively were revealed by ECL. Western blotting with OM specific anti-OmpA (lanes 1–5, OmpA: 35 kDa and a smaller degraded form) or with IM specific anti-LepB (lanes 4, 5, LepB: 36 kDa) antiserums showed (lower parts of the panel) that the membrane fractionation was efficient. Periplasmic extracts (lanes 6, 8, 10, PE) were also analysed with anti-colicin E2 (lanes 6, 7) and anti-OmpA (lanes 8, 9, OmpA: including some degraded OmpA forms) or anti-MalE that detects the periplasmic maltose-binding protein (lanes 10, 11, MBP: ∼43 kDa) antiserums. Proteinase K (PK) treatment (100 µg/ml; 1 h) is systematically applied to colicin-treated cells. (B) CE of OmpT-deficient cells not treated (lanes 3, 6) or treated with colicin E7-ImmE7 (lanes 1, 4, 7–9) or with colicin E2-ImmE2 (lanes 2, 5) were analysed in parallel by Western blotting with anti-colicin E2 or anti-colicin E7 antiserum. PK concentrations (µg/ml) are only indicated when higher concentrations of PK were compared to standard PK concentration of 100 µg/ml (lanes 7–9) and some MAF levels were quantified and expressed as a percentage of MAF value, measured with the standard PK concentration (lane 9, 100%). The molecular weights (MW) are given in kDa. (C) Identification of a MAF-like peptide from colicin E3-ImmE3 (lanes 5, 6, E3) treated bacteria after a 12-fold reduction in the time of hydrolysis (t in min) with PK, in comparison with the endoproteolytic cleavage profile detected from colicin E2-ImmE2 treated cells (lanes 2, 3, E2). Analysis of colicin-treated bacteria without PK hydrolysis is shown (t = 0, lanes 1, 4). Proteins of crude cell extracts were separated and analysed by Western blotting with anti-colicin E2 or anti-colicin E3 antiserum.