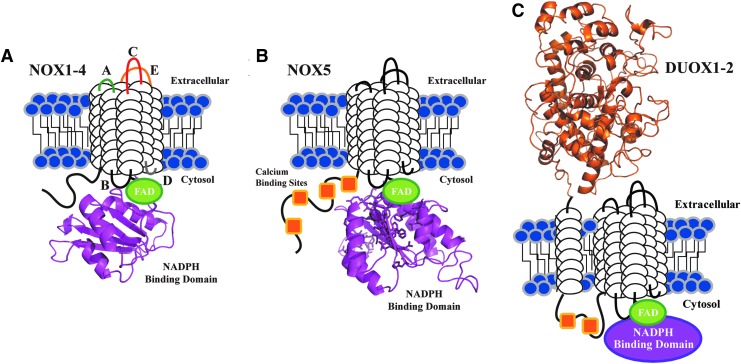
FIG. 2.
Schematic view of the conserved structural features of the NADPH oxidase family. Each NOX/DUOX isoform contains six putative TM domains (white cylindrical loops), with C-terminal FAD (green) and NADPH binding domains (purple). The NADPH binding domain structural models were created by the SWISS-MODEL program server with hNOX2 (PDB: 3A1F) as the template. A model of the peroxidase-like domain of DUOX2 is also highlighted and was created as previously reported (92); all models were visualized by Pymol software. (A) NOX1-4 isoforms are depicted with loop regions labeled based on established designations: extracellular loops A (green), C (red), E (yellow) and intracellular loops B (black) and D (gray). (B) Each NOX5 isoform contains four N-terminal calcium binding sites (orange squares); the hNOX2 and hNOX5 NADPH binding domains share 36% amino acid sequence identity. Amino acids displayed as sticks represent the predicted NADPH binding site residues. (C) DUOX1-2 are unique to the NADPH oxidase family, as both isoforms contain an extracellular N-terminal peroxidase homology domain (orange) tethered to a TM domain, and two cytosolic calcium binding sites.