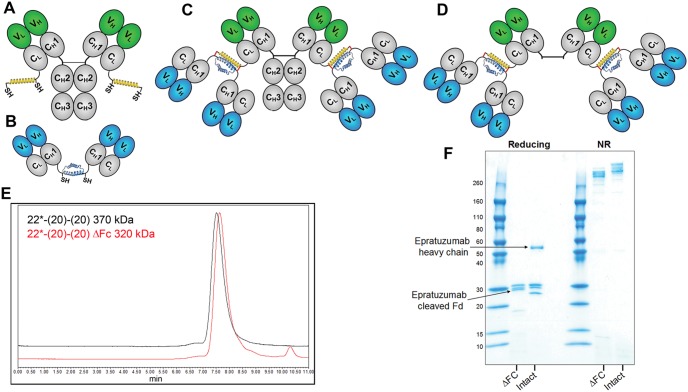
Figure 1. DNL modules and bsHexAb structures.
(A) Ck-AD2-IgG-epratuzumab, an IgG-AD2 module with an AD2 fused to the carboxyl-terminal end of each kappa light chain. (B). Dimeric CH1-DDD2-Fab-veltuzumab, or CH1-DDD2-Fab-hA9, Fab-DDD modules with DDD2 fused to the carboxyl-terminal end of the Fd chain (C). Structure of 22*-(20)-(20) or 22*-(19)-(19), bsHexAbs comprising Ck-AD2-IgG-epratuzumab and two dimeric CH1-DDD2-Fab-veltuzumab or CH1-DDD2-Fab-hA19 modules, respectively. (D) Structure of 22*-(20)-(20) with the Fc removed. Variable (V, blue or green) and constant (C, grey) domains of IgG heavy (H) and light (L) chains are represented as ovals. The DDD2 (dimerization and docking domain) and AD2 (anchor domain) peptides are shown as blue and yellow helices, respectively, with the locations indicated for the reactive sulfhydryl groups (SH) and the “locking” disulfide bridges indicated as red lines. (E) SE-HPLC showing the homogeneity of 22*-(20)-(20) and the expected small shift in retention following removal of the Fc, which comprises 13% of the protein. (F) Reducing (left) and non-reducing (right) SDS-PAGE showing the elimination of the intact epratuzumab heavy chain (intact lane) and the appearance of the resulting cleaved epratuzumab Fd following removal of the Fc (ΔFc lane).