Figure 7. Scheme of how PKD subtypes may contribute to regulate cofilin activity and directed cell migration.
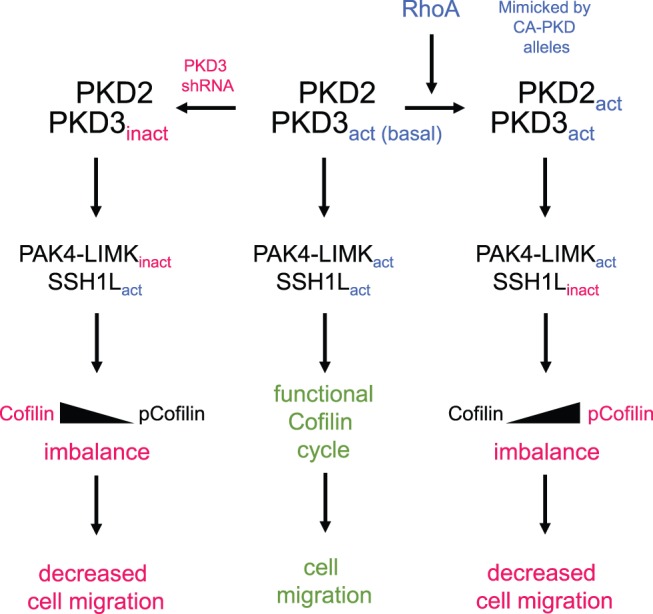
Using HeLa cells, which only express the PKD subtypes PKD2 and PKD3 as a model system, we suggest the following scheme: In cells under normal growth conditions (middle), PKD2 and PKD3 form a complex in which PKD3 is basally active. Basal activity of PKD3 contributes to PAK4/LIMK activity. Together with active (unphosphorylated) SSH1L, active PAK4 guarantees a functional cofilin activity cycle and cell migration. If PKD3 is inactivated or decreased in its expression (left side), PAK4 activity decreases, while SSH1L stays active. Net effect is an increase in non-phosphorylated (active) cofilin. This leads to imbalanced F-actin severing and decreased cell migration. On the other hand, when PKD2 and PKD3 are further activated (i.e. by activation of RhoA) they phosphorylate SSH1L at S978, which leads to its inactivation (right side). In this scenario, basal PAK4 activity and decreased SSH1L activity increase phospho-cofilin levels dramatically, preventing F-actin severing and directed cell migration.
