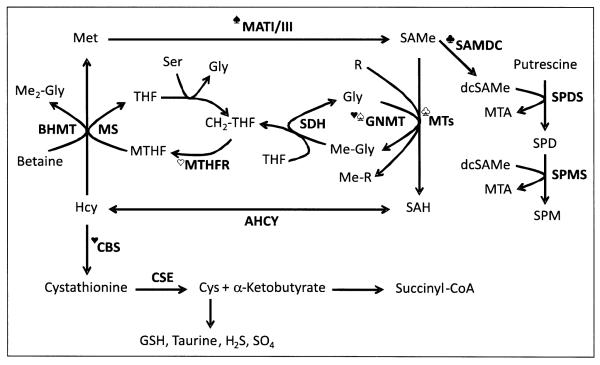
Figure 1.
Hepatic S-adenosylmethionine (SAMe) metabolism. Methionine (Met) is an essential sulfur-containing amino acid that is converted to SAMe by the enzyme methionine adenosyltransferase (MAT) using ATP as co-substrate. Two main MAT isoforms, MATI and MATIII, are expressed in the liver. MATIII is activated by methionine. SAMe’s methyl group is transferred to a large variety of substrates, such as DNA, RNA, proteins, phosphatidylethanolamine, glycine (Gly), and guanidinoacetate. These reactions receive the general name of transmethylation reactions, and are catalyzed by specific methyltransferases (MTs). S-adenosylhomocysteine (SAH), the byproduct of att transmethylation reactions, is an inhibitor of MTs. SAH is hydrolyzed to form homocysteine (Hcy) and adenosine by a reversible enzyme known as SAH hydrolase (AHCY). Hcy may be remethylated to regenerate Met, or enter the transsulfuration pathway to be converted first to cystathionine, by a condensation reaction with homoserine, and then to cysteine (Cys) and α-ketobutyrate. Whereas Cys is transformed into a variety of sulfur containing molecules, such as glutathione (GSH), taurine, sulfate (SO,4 and hydrogen sulfide (H2S), α-ketobutyrate is transformed to succinyl-CoA and metabolized in the mitochondria. Cystathionine β-synthase (CBS), the enzyme that catalyzes the synthesis of cystathionine, is activated by SAMe. Methionine is regenerated from Hcy via two routes; the methionine synthase (MS) pathway, which uses 5-methyttetrahydrofolate (MTHF) as methyl donor, and the BHMT route, that uses betaine (trimethylglycine) as methyl donor. The synthesis of MTHF is catalyzed by the enzyme MTHF reductase (MTHFR), which is inhibited by SAMe. In its turn, MTHF inhibits GNMT, the enzyme that converts Gly to sarcosine (methylglycine, Me-Gly). GNMT is activated by SAMe. The regeneration of Gly from sarcosine is catalyzed by the enzyme sarcosine dehydrogenase (SDH).
Another major role of SAMe is to donate its propylamino group for polyamine synthesis. Here, SAMe is first decarboxylated by the enzyme SAMe decarboxylase (SAMDC) to form decarboxylated SAMe (dcSAMe). Then, this compound donates its propylamine group to putrescine to form spermidine (SPD), and to SPD to form spermine (SPM). This pathway is regulated by putrescine, which activates SAMDC. Methytthioadenosine (MTA), a byproduct of SPD and SPM synthesis, is used to regenerate methionine (not shown). Ser, serine; SPDS, SPD synthase; SPMS, SPM synthase; THF, tetrahydrofolate; CH2-THF, methylene tetrahydrofolate; R, methyl accepting substrate; Me-R, methylated product; Me2-Gly, dimethylglycine.  , activated by methionine;
, activated by methionine;  , activated by putrescine;
, activated by putrescine;  , activated by SAMe;
, activated by SAMe;  , inhibited by SAMe;
, inhibited by SAMe;  , inhibited by MTHF;
, inhibited by MTHF;  , inhibited by SAH. Metabolites and enzymes are indicated by normal and bold letters, respectively.
, inhibited by SAH. Metabolites and enzymes are indicated by normal and bold letters, respectively.