Scheme 1. Synthesis of A-Ring Precursors 2a,b.
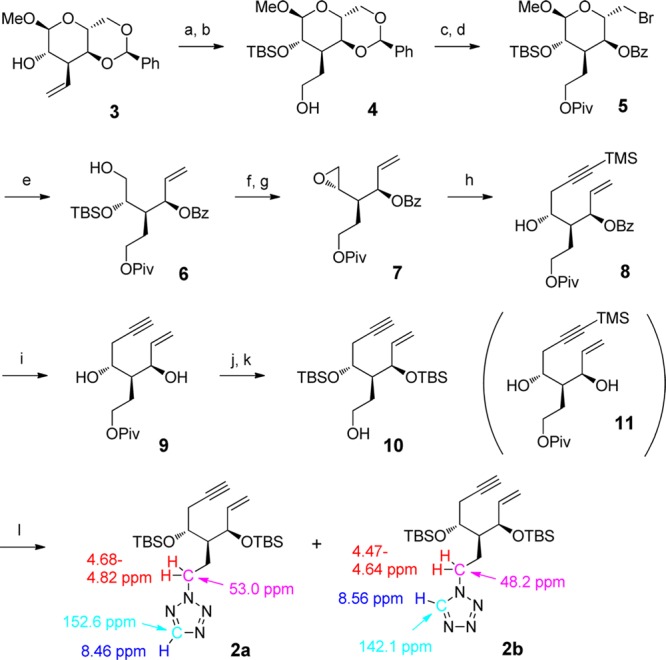
Reagents and conditions: (a) TBSOTf, 2,6-lutidine, CH2Cl2, 85%. (b) 9-BBN, THF, and then, H2O2, aq. NaOH, 92%. (c) PivCl, py, CH2Cl2, 95%. (d) NBS, BaCO3, CCl4, 87%. (e) Zn powder, NaBH3CN, 1-PrOH–H2O (5:1), 70%. (f) TsCl, py, 96%. (g) TBAF, THF, 80%. (h) BuLi, TMS-acetylene, BF3·OEt2, THF, 78%. (i) K2CO3, MeOH, 71%. (j) TBSOTf, 2,6-lutidine, CH2Cl2, 93%. (k) DIBAL-H, CH2Cl2, 92%. (l) DIAD, PPh3, 1H-tetrazole, THF, 2a 81% and 2b 19%.
