Figure 5.
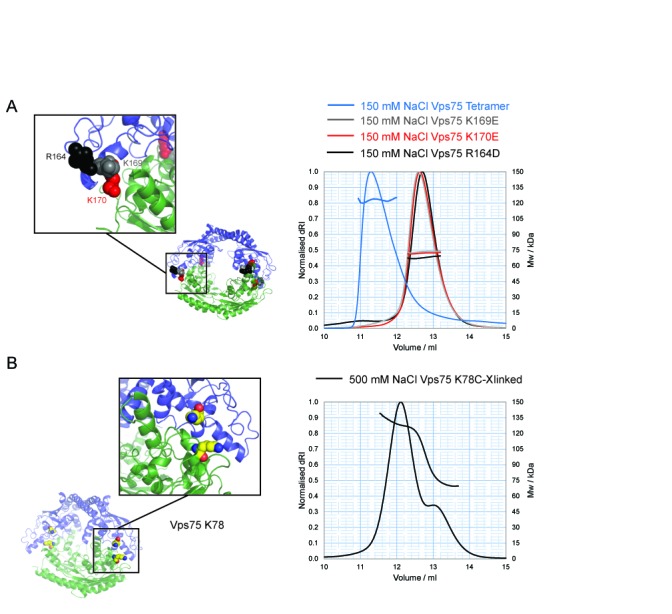
Structure-based mutagenesis to disrupt the Vps75 tetramer. (A) Model of Vps75 tetramer, opposing Vps75 dimers in blue and green, with the positions of non-tetramerising mutants shown as colour-coded spheres matching SEC–MALS elution profiles of the corresponding mutant; K169E (grey), K170E (red) and R164D (black) compared to wild-type Vps75 (blue). (B) The location of K78 (yellow spheres) that was mutated to cysteine for disulphide cross-link formation to trap Vps75 in its tetrameric conformation and SEC–MALS analysis at 500 mM NaCl of Vps75 disulphide cross-linked at 150 mM NaCl via K78C.
