Figure 7.
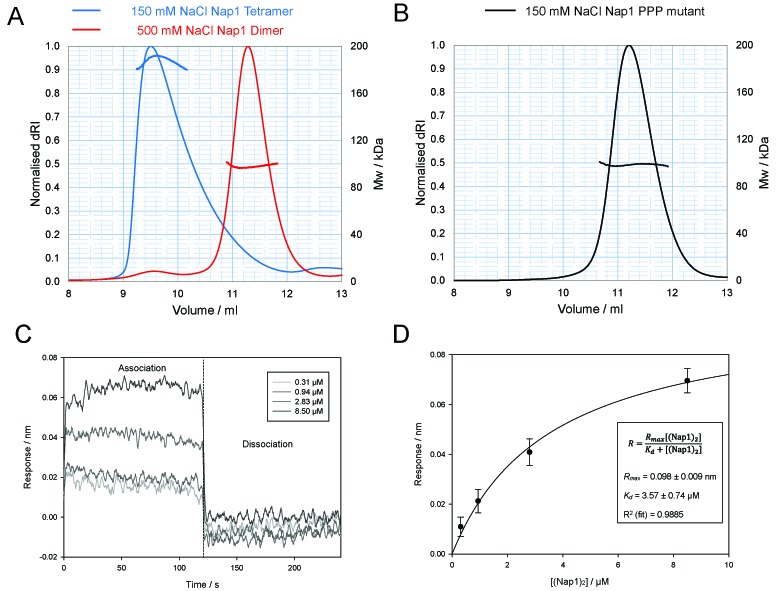
SEC–MALS analysis of the structurally related protein Nap1 suggests it also adopts a stable tetramer mediated by the β-hairpin. (A) SEC–MALS elution profiles of Nap1 at 150 mM (blue) and 500 mM NaCl (red). (B) SEC–MALS analysis of the Nap1 PPP mutant. The molar mass over elution peaks is shown in corresponding colours. The association of soluble Nap1 with Nap1 dimers immobilized to a probe via a biotin linkage was measured using bio-layer interferometry. Changes in the response units were measured in the presence of increasing concentrations of soluble Nap1 dimers (C). A fit to the binding response data was then used to calculate the dissociation constant, Kd (D).
