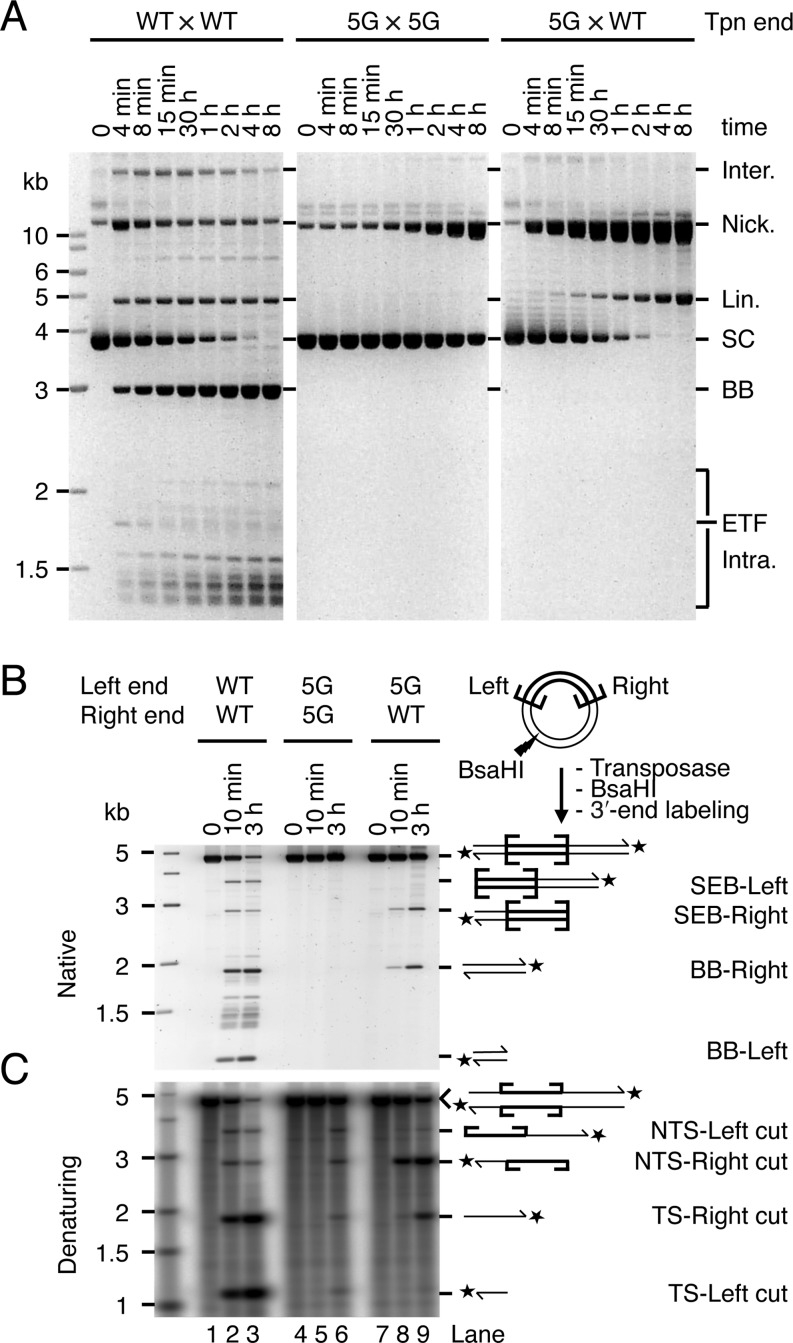
Figure 3.
The two transposon ends are not independent during catalysis. (A) The kinetics of transposition reactions with a supercoiled substrate that carried two wild-type transposon (Tpn) ends (WT × WT), two 5G mutant transposon ends (5G × 5G) or a 5G mutant and a wild-type end (5G × WT) were analyzed by native agarose gel electrophoresis. The intermediates and products of these reactions are as illustrated in Figure 1A, except that the samples were deproteinated before loading the gel. The identity of these products has been determined previously by restriction digestion analysis, one- and two-dimensional gel electrophoresis, and DNA sequencing (8,14,26). The 5G mutant has an array of five guanine nucleotides spanning from position −2 to +3 on the NTS (see numbering in Figure 1C). (B, C) The products of transposition reactions with the substrates used in part A were digested with the restriction enzyme BsaHI, 3′-labeled with α-32P-dCTP and the Klenow enzyme and analyzed by native (B) and denaturing (C) agarose gel electrophoresis. (B) A SYBR Green I stained 1.1% TBE-based agarose gel is shown. (C) The autoradiogram of a 1.5% alkaline agarose gel is shown.