Figure 1.
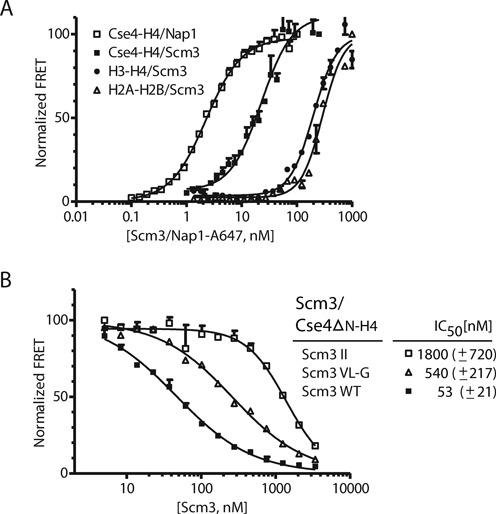
Scm3 binds to Cse4–H4 with nanomolar affinity. (A) Affinities of Scm3 for various histone complexes were measured using the HI-FI assay (50). ‘Histone complexes, as indicated, were labeled with Alexa-488 (donor, held constant at 1–4 nM), and incubated with increasing amounts of Atto-647N labeled Scm3 or Nap1 (acceptor) and scanned for donor, acceptor and FRET fluorescent signal. Representative binding curves are shown; all data are summarized in Table 1. (B) Competition assay to measure the relative affinity of wild-type and Scm3 mutants. Acceptor labeled Scm3 and donor labeled Cse4ΔN–H4 were kept constant at 50 and 10 nM, respectively. Unlabeled wild-type or mutant Scm3 was titrated and the FRET signal was monitored. Wild-type Scm3: solid squares (scm3 WT); double Scm3 mutations I111D I117N (Scm3 II): open squares; triple Scm3 mutations V158G L159G I161G (Scm3 VL-G): open triangles. The inset lists the derived IC50 values with standard deviations; derived from at least three independent experiments.
