Figure 3.
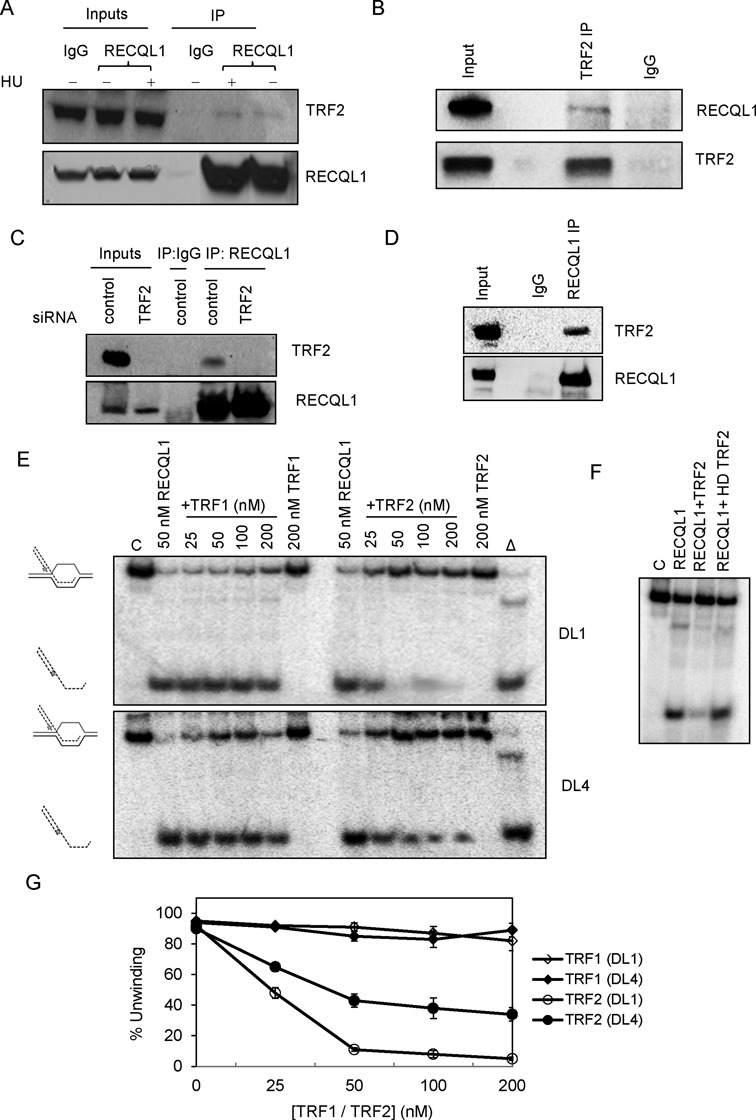
RECQL1 physically and functionally interacts with TRF2. (A) Co-immunoprecipitation (co-IP) of RECQL1 and TRF2 from whole cell extracts of U2OS cells, performed in the presence of ethidium bromide, both in the presence and absence of HU (treated with 5 mM for 18 h). (B) Reverse co-IP was performed with the antibody against TRF2 and blotted for RECQL1. (C) Co-IP of RECQL1 and TRF2 from whole cell extracts of U2OS cells transiently depleted with siRNA against TRF2. All the co-IP assays were performed in the presence of ethidium bromide to ensure that the interaction is not DNA mediated. (D) In vitro co-IP performed using both the purified proteins TRF2 and RECQL1. (E) Helicase activity of RECQL1 (50 nM) performed in the presence of increasing concentrations of either TRF1 (25–200 nM) or TRF2 (25–200 nM) using an undamaged telomeric D-loop (DL1) or a damaged telomeric D-loop containing 8-oxoguanine (DL4). (F) Effect of heat denatured (HD) TRF2 on the helicase activity of RECQL1 using the telomeric D-loop. (G) Plot showing the effect of increasing concentrations of both TRF1 and TRF2 on the helicase activity of RECQL1.
