Abstract
The original life-history strategy of brood-parasitic birds has been the focus of a large number of studies in ecology and evolution. Whether species adopting such a strategy differ in their response to global changes remains, however, unknown. Both the absence of investment in parental care and the capacity to spread nesting failure by laying eggs in several nests might help brood parasites in dealing with environmental changes. Alternatively, brood parasites might cumulate the negative effects of environmental changes on their own environment and on their hosts’ environment. Here, I tested whether brood parasites’ extinction risk and population trend differed from those of species with parental care. Focusing on the five bird families containing brood parasite species, I show that brood parasites are less at risk of extinction, and have a more stable population trend than species with parental care. In addition, I found that brood parasites with a higher host diversity were more likely to be increasing than those with fewer hosts. The bet-hedging strategy of brood parasites, by allowing them to spread nesting failure risks associated with environmental changes, is likely to help them resist current global changes.
Keywords: bet-hedging, International Union for Conservation of Nature Red List, global changes, host generalism
1. Introduction
In conservation biology, avian brood parasitism is, sometimes, seen as a potential threat for the host species. Some brood parasites, especially cowbirds, have, indeed, been shown to have negative effects on the reproductive success of host populations and species, eventually increasing their extinction risk [1,2]. However, it is currently unknown whether brood parasitism confers advantages to the parasite itself in the face of the current acceleration in global challenges to biodiversity. Payne [3, p. 13] has observed that ‘no brood-parasitic cuckoos appears on the list of threatened and endangered birds’, whereas some non-parasitic cuckoos are considered at risk of extinction. Obligate brood-parasitic birds (hereafter called brood parasites) do not display parental care, but rely on their hosts to breed their chicks. Both the absence of investment in parental care and the capacity to lay eggs in several nests, spreading nesting failure risk (bet-hedging strategy [3–5]), might help brood parasites limit the effects of environmental changes on their survival and reproduction. Alternatively, however, the dependence of brood parasites on host species for reproduction might lead to an increased effect of environmental changes on brood-parasitic species, as both changes in their own environment and changes in their hosts’ environment might affect their fitness.
Investigating the effects of brood parasitism on diversification rates within Cuculidae, Krüger et al. [6] showed that brood-parasitic Cuculidae display both a higher speciation rate and a higher extinction rate than species with parental care, resulting in a similar net diversification rate. The high extinction rate of brood parasites was mainly attributed to the arms race between hosts and parasites, some hosts evolving defences that a parasite cannot overcome, thus leading to the extinction of their associated parasitic species [7,8]. However, extinction rate in the past is not necessarily a good predictor of resistance to current anthropogenic environmental changes, as the causes of extinction are likely to be different. Whether brood parasite species differ from species with parental care in their response to current global changes thus deserves direct investigation.
Among brood parasite species, parasitism strategies can be very different. Some species, such as most of the Viduidae (Indigobirds and Whydahs), are host specialists, exclusively parasitizing one host species. Others, such as the common cuckoo, Cuculus canorus, lay their eggs in the nests of more than 100 different host species. In the same way as habitat generalist species are less affected by global changes than habitat specialists [9,10], we might expect host-generalist brood parasites to resist better than host specialists. Host generalism can also be seen as a bet-hedging strategy, as the reproductive success of host generalists, at the individual, population or species level, will not exclusively depend on one host species: the decrease in one or some host populations should have a lower impact on host-generalist brood parasites than on host specialists. However, whether host diversity predicts brood parasites' response to current global changes remains unknown.
The aim of this study was first to test whether brood parasite species differ from species with parental care in their response to global changes. I focused my analyses on the five families containing brood parasite species, and used the International Union for Conservation of Nature (IUCN) extinction risk classification as well as population trend data as proxies of response to current global changes. Focusing on brood parasite species, I then tested whether responses to global changes depend on host diversity.
2. Material and methods
(a). Dataset
I conducted the analyses on the families containing obligate interspecific brood parasite species in order to compare brood parasite species and species with parental care within these families. I extracted the list of brood parasites and their hosts from Peter Lowther's website at http://fieldmuseum.org/explore/brood-parasitism-host-lists (Field Museum, Chicago, IL, USA). I then excluded species not recognized by the IUCN Red List nomenclature as well as data-deficient species. My final dataset featured five families known to contain brood parasites, i.e. Anatidae, Cuculidae, Icteridae, Indicatoridae and Viduidae, a total of 430 species of which 96 are obligate brood parasites.
Host diversity data were available for 80 of the 96 parasitic species. I first calculated the total number of different host species recorded for each parasite species as an index of host diversity. As some of these species are poorly documented as hosts or are unusual hosts potentially resulting from host choice errors, I developed a second index excluding poorly supported cases. This second index had such a high correlation with the first one (Spearman's ρ = 0.983; p < 0.001, n = 80) that all the analyses reported here were performed with the first index. As the number of host species recorded by ornithologists is likely to be higher for more intensely studied species, I measured the research effort invested in each one as the number of papers published on each species between 1978 and 2008 according to the online version of the Zoological Record. As predicted, research effort and host diversity were, indeed, positively correlated (Spearman's ρ = 0.537; p < 0.001), so I expressed host diversity as the residual of a (log-link) quasi-Poisson regression with host diversity as response variable and research effort (log-transformed) as explanatory variable.
I used the IUCN Red List status as a measure of species extinction risk. I converted the threat categories to an ordinal index from least concern (1) to critically endangered (5). A species extinction risk is not only assigned according to a species population state (such as its population and distribution size), but also by a species population trend. I also separately considered the population trend as given by the IUCN, converting it into an ordinal index (1, increasing; 2, stable; 3, decreasing). Population trend was available for 416 species including 93 brood parasites.
(b). Confounding variables
I incorporated in the analyses potential confounding traits that differ between brood parasites and species with parental care and that are likely to affect responses to global change. I included body mass, generation length, migratory behaviour, insularity, distribution range and habitat generalism in the analyses. Mass data were available from reference [11]. I extracted generation length, migratory behaviour, insularity and distribution range (in km²) from the IUCN and Birdlife websites (http://www.iucnredlist.org/; http://www.birdlife.org/datazone/species). Migratory behaviour was coded as a categorical variable, with 1, sedentary or nomadic, 2, altitudinal migrant and 3, long distance migrant, and insularity as a binary variable, with 0 for continental species and 1 for insular species. To estimate habitat generalism, I calculated the number of different habitat classes each species inhabits [12,13]. The IUCN provides a habitat classification scheme that defines 82 different habitat subtypes. I assigned each IUCN habitat into one of six categories (forest, scrub, wetland, cultivated and farm lands, marine, urban), and summed for each species the number of different categories it was recorded in, from 1 to 6. The whole dataset is available as the electronic supplementary material, S1.
(c). Analyses
I used phylogenetic generalized linear-mixed models with Markov chain Monte Carlo (MCMC) techniques using the R package MCMCglmm. To test for differences in responses to global change (IUCN status or population trend) between brood parasites and species with parental care, I first fitted models that included all fixed effects as explanatory variables, and then used backward selection to identify minimal adequate models that retained only significant variables. Initially included fixed effects were breeding system (binary variable distinguishing between brood parasites and species with parental care), body mass, generation length, insularity, migratory behaviour and habitat generalism. As suggested by Hadfield [14], I used ordinal distribution for all models as I had ordinal response variables (IUCN status or population trend). Biogeographic area (10 categories: Africa, Antarctica, Asia, Australasia, Caribbean, Central America, Europe, North America, South America plus one category for species occupying two or more realms) was included as a random effect. To take phylogeny into account, I used the phylogeny from Jetz et al. [15] available at http://birdtree.org/ and included it as a random effect. This website does not provide one unique consensus tree, but samples trees from a pseudo-posterior distribution. I first conducted the model selection five times independently using five randomly extracted trees. Model selection always yielded the same minimum adequate model, whichever tree was considered. Then, I extracted 50 different trees and ran the selected model considering each of the 50 trees. I then averaged the model parameters over the 50 different phylogenies. For all models, the MCMC chains were run for 210 001 iterations with a burn-in interval of 10 000 to ensure satisfactory convergence. A total of 1000 iterations were sampled to estimate parameters for each model. I verified that autocorrelation levels among samples were lower than 0.1. According to Hadfield [14], I fixed the covariance structure and used poorly informative priors for the variances.
All analyses were run on five different datasets to test whether the results were driven by some specific families. I first included all species as described above, then excluded either Anatidae as this family is large (168 species), but contains only one obligate interspecific brood parasite (Heteronetta atricapilla), or both Viduidae and Indicatoridae as these families are exclusively made of brood parasite species. The Cuculidae family contains more than 50% of all brood parasite species. I thus also conducted analyses excluding Cuculidae from the dataset, or focusing exclusively on the Cuculidae family. As the analyses on the five datasets yielded qualitatively similar results with regards to the effect of breeding system, only results with the whole dataset are shown in the main text (see the electronic supplementary material, S2 and S3, for details of results with the other datasets). A challenge for comparative models that use Red List status to assess vulnerability is that testing whether range area determines vulnerability is a circular argument for species listed as threatened because of their small range. I thus conducted the analyses twice: first without the distribution range, then including it as a fixed variable. As including or not the distribution range did not affect the results with regards to the effect of breeding system, I only show the models that include it.
In order to test whether host diversity affected brood parasite response to global changes, I focused on the 80 brood parasite species for which I had host data, and built models with the same fixed and random effects as previously described, but added host diversity (corrected by research effort) instead of breeding system. I used the procedures previously described to build and select models and to deal with phylogenetic uncertainty.
In all analyses, excluding phylogeny or biogeographic area from the models largely increased the deviance information criterion (ΔDIC > 213, where the DIC is a hierarchical modelling generalization of the Akaike information criterion and Bayesian information criterion currently used in Bayesian model selection problems where the posterior distributions of the models have been obtained by MCMC simulation (cf. [14]). I thus always included both biogeographic area and phylogeny as random factors.
3. Results
(a). Response to global changes in brood parasites versus species with parental care
Brood parasites had a significantly lower extinction risk than species with parental care (pMCMC = 0.007, table 1 and figure 1). Among the 96 brood parasite species, four are considered as ‘near threatened’, and the 92 others are classified as ‘least concern’ (figure 1). Within families containing brood parasite species, the extinction risk of species with parental care is distributed as follows: 267 are classified as ‘least concern’ and 67 are distributed among the four other threat categories (figure 1). Habitat generalist species had a lower extinction risk (pMCMC = 0.005) and insular species faced a higher extinction risk than continental ones (pMCMC = 0.002). Species with a larger distribution range were less at risk of extinction (pMCMC = 0.001). By contrast, generation length, body mass and migratory behaviour did not affect extinction risk (pMCMC > 0.234).
Table 1.
Best model explaining avian extinction risk as a function of breeding system (brood-parasitic species versus species with parental care) and covariables in families containing brood-parasitic species. Best models were run with 50 different phylogenies, and posterior means (pm), confidence interval (CI) and p-values were averaged over the 50 models with 50 different phylogenetic trees (see text for details). Extinction risk was classified from least concern (1) to critically endangered (5), so that a negative pm indicates decreasing extinction risk. Species with parental care and continental species were taken as references for the calculation of coefficients.
| variable | pm | CI | pMCMC |
|---|---|---|---|
| breeding system | −1.558 | [−2.822; −0.368] | 0.007 |
| habitat generalism | −0.343 | [−0.588; −0.099] | 0.005 |
| insularity | 0.985 | [0.336; 1.646] | 0.002 |
| distribution range | −3.847 × 10−7 | [−5.567 × 10−7; −2.154 × 10−7] | 0.001 |
Figure 1.
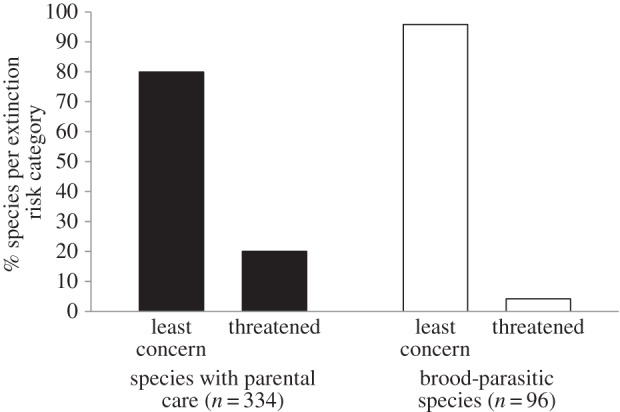
Distribution of brood parasites and species with parental care among extinction risk categories. Threatened species (critical, endangered, vulnerable and near threatened) were grouped into a single category. Species from the five families containing obligate brood parasites are included.
Population trends were also significantly different between brood parasites and species with parental care (pm = −54.287; CI = [−139.808; 3.965]; pMCMC = 0.041; figure 2). Indeed, 40.6% of parental care species showed decreasing trends, whereas only 16.1% of brood parasite populations were decreasing. Most of the brood parasites had a stable trend (78.5% versus 46.1% in species with parental care), whereas the proportion of species with increasing trends was lower in brood parasites (5.4%) than in species with parental care (13.3%; figure 2). Habitat generalism also predicted species population trends, as habitat generalists were less likely to be decreasing (pm = −28.279; CI = [−65.680; −1.035]; pMCMC = 0.009); none of the other tested factors significantly explained population trends (pMCMC > 0.154).
Figure 2.
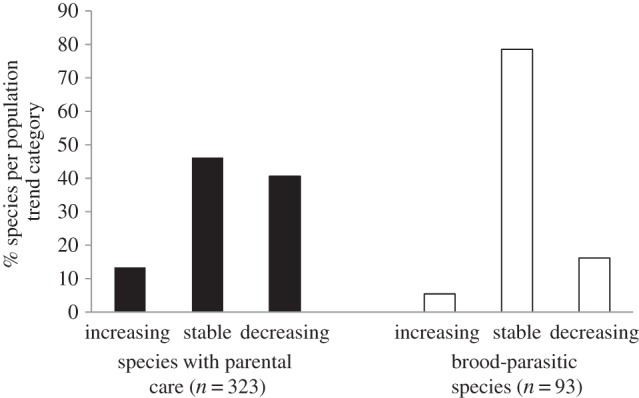
Distribution of brood parasites and species with parental care among population trend categories. Species from the five families containing obligate brood parasites are included.
(b). Host diversity and population trend
Within brood-parasitic species, variation in extinction risk was too low to test for an effect of host diversity: host diversity was available for 79 species with ‘least concern’ status, and only one species with ‘near threatened’ status (Cuculus vagans, with three different known hosts). I thus tested only for an effect of host diversity (corrected by research effort) on population trends. Host diversity significantly predicted population trends, brood parasite species with a larger diversity of hosts being more likely to be on the increase (figure 3; pm = −0.531; CI = [−1.133; −0.057]; pMCMC = 0.001). All other tested factors were not significant and were thus excluded from the model. Note that habitat generalism and distribution range were positively correlated with host diversity (Spearman's correlation: ρ = 0.312; p = 0.016 for habitat generalism; ρ = 0.479; p < 0.001 for distribution range). Habitat generalism and distribution range, however, did not explain population trends, even when testing their effect using univariate analyses (pMCMC = 0.133 for habitat generalism, pMCMC = 0.289 for distribution range).
Figure 3.
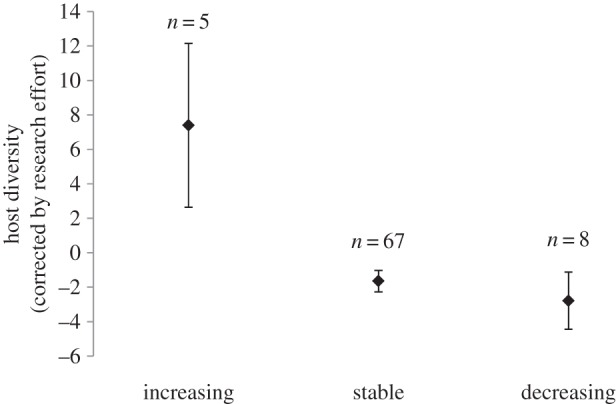
Mean host diversity (residuals of a quasi-Poisson regression of host number versus research effort) of species within each population trend category (mean ± s.e.).
4. Discussion
These results clearly show that brood parasites are less at risk of extinction than species with parental care. This conclusion was robust when testing for potential confounding variables such as phylogeny, biogeographic area, habitat generalism, distribution range and several life-history traits. In addition, the fact that I obtained similar results when excluding Anatidae, Cuculidae or Viduidae and Indicatoridae, or when focusing only on Cuculidae, also strengthens these results, showing that the lower extinction risk of brood parasites is a general pattern not limited to one family. This difference in extinction risk is confirmed by non-parasite species being more likely to have decreasing population trends than brood parasite species (figure 2), although the higher stability of brood parasite populations might also be due to the difficulties in monitoring parasite species owing to their unusual life history, leading ornithologists to categorize them stable rather than decreasing or increasing. Consistent with other studies, habitat generalists, continental species and species with a larger distribution range were also less at risk of extinction [9,10,16].
These analyses thus show that traits such as habitat generalism or distribution range are not the only factors explaining the better resistance to global changes of brood parasites, suggesting that the parasitic strategy per se might be advantageous in facing current global changes. Brood parasites usually lay their eggs in a large number of different nests (of one or several species), thus spreading nest failure risk. This bet-hedging strategy might help brood parasites limit the effects of environmental changes. Indeed, bet-hedging strategies are expected to be adaptive when environmental conditions are changing or unpredictable [17]. This hypothesis, however, calls for a more detailed investigation of whether and how bet-hedging strategies, in general, can help species respond to current global changes. For example, spreading nest failure risks through intraspecific parasitism [18] or by laying several clutches per reproduction season might be advantageous under current global changes, although this needs to be tested. Alternatively, the finding that brood parasite species resist global changes better might result from the brood parasitism strategy being linked to some level of ecological flexibility. Such flexibility, expressed through habitat, food and/or behavioural flexibility, might indeed confer advantage in varying environment. Whether or not brood-parasitic species display a higher ecological flexibility remains, however, to be investigated.
The finding that host diversity predicts population trends within brood-parasitic species also suggests that laying eggs in the nests of a high diversity of species is advantageous. Brood parasite population trends are indeed expected to rely directly on host population trends. Host generalists might then be better able to attenuate the effect of a given host population decrease, which can also be considered a bet-hedging strategy. This result thus tends to confirm the hypothesis that spreading nest failure risk by laying eggs in several nests, but also in the nests of different species, might be advantageous under current global changes. Within host-generalist species, some are known to be generalist at the species but not the individual level (e.g. the common cuckoo, C. canorus, where most females use one specific host species despite the species being host-generalist, a strategy known as ‘gentes’), whereas others, such as the brown-headed cowbird, Molothrus ater, are generalists at both the species and individual levels. Testing whether species with gentes respond differently to environmental changes from species with host-generalist individuals might help detail the importance of bet-hedging strategies under current global changes. A better knowledge of brood parasites' life history is, however, required before we are able to conduct such an analysis.
The effect of host diversity on population trends might also arise from a better ability to switch from one host to the other in host-generalist species. A brood parasite's host diversity might, indeed, reflect its past ability to switch from one host species to another in response to some hosts developing anti-parasite defences, or to a decrease in the host population. Disentangling the relative importance of variation in host diversity versus variation in the ability to switch to new hosts might help us understand the evolutionary and ecological mechanisms behind variation in population trends among brood parasites.
Coevolutionary theory predicts that brood parasites will become more specialized the longer they are in contact with a particular avifauna [19,20]. By contrast, current global changes may favour the maintenance of a generalist strategy by decreasing avian community stability, favouring bet-hedging strategies and limiting host specialization. Whether or not current environmental changes will overcome the coevolutionary arms race driving brood parasites towards specialization will likely depend on the strength and duration of the changes. The arms race coevolution between host and parasites thought to have led to the current brood parasites diversity could thus be deeply affected by current global changes.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
I thank Peter Lowther for making his brood parasites host database available, and warmly thank Louis Lefebvre, Oliver Krüger and an anonymous reviewer for suggestions that greatly improved the manuscript.
Funding statement
This work was supported by a post-doctoral fellowship from the Fondation Fyssen to S.D. and an NSERC Discovery grant to Louis Lefebvre.
References
- 1.Fenton A, Rands SA. 2006. The impact of parasite manipulation and predator foraging behavior on predator–prey communities. Ecology 87, 2832–2841. ( 10.1890/0012-9658(2006)87[2832:TIOPMA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 2.Lefèvre T, Lebarbenchon C, Gauthier-Clerc M, Missé M, Poulin R, Thomas F. 2009. The ecological significance of manipulative parasites. Trends Ecol. Evol. 24, 41–48. ( 10.1016/j.tree.2008.08.007) [DOI] [PubMed] [Google Scholar]
- 3.Payne R. 2005. The cuckoos. Oxford, NY: Oxford University Press. [Google Scholar]
- 4.Slatkin M. 1974. Hedging one's evolutionary bets. Nature 250, 704–705. ( 10.1038/250704b0) [DOI] [Google Scholar]
- 5.Brown CR, Brown MB. 1996. Coloniality in the cliff swallow, the effect of group size on social behavior. Chicago, IL: University of Chicago Press. [Google Scholar]
- 6.Krüger O, Sorenson MD, Davies NB. 2009. Does coevolution promote species richness in parasitic cuckoos? Proc. R. Soc. B 276, 3871–3879. ( 10.1098/rspb.2009.1142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies NB. 2000. Cuckoos, cowbirds and other cheats. London, UK: T. & A. D. Poyser. [Google Scholar]
- 8.Honza M, Procházka P, Stokke BG, Moksnes A, Røskaft E, Čapek M, Mrlík V. 2004. Are blackcaps current winners in the evolutionary struggle against the common cuckoo? J. Ethol. 22, 175–180. ( 10.1007/s10164-004-0119-1) [DOI] [Google Scholar]
- 9.Jiguet F, Gadot AS, Julliard R, Newson SE, Couvet D. 2007. Climate envelope, life history traits and the resilience of birds facing global change. Glob. Change Biol. 13, 1672–1684. ( 10.1111/j.1365-2486.2007.01386.x) [DOI] [Google Scholar]
- 10.Lee TM, Jetz W. 2011. Unravelling the structure of species extinction risk for predictive conservation science. Proc. R. Soc. B 278, 1329–1338. ( 10.1098/rspb.2010.1877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunning JB. 2008. CRC handbook of avian body masses, 2nd edn Boca Raton, FL: CRC Press. [Google Scholar]
- 12.Sol D, Duncan RP, Blackburn TM, Cassey P, Lefebvre L. 2005. Big brains, enhanced cognition, and response of birds to novel environments. Proc. Natl Acad. Sci. USA 102, 5460–5465. ( 10.1073/pnas.0408145102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Overington SE, Griffin AS, Sol D, Lefebvre L. 2011. Are innovative species ecological generalists? A test in North American birds. Behav. Ecol. 22, 1286–1293. ( 10.1093/beheco/arr130) [DOI] [Google Scholar]
- 14.Hadfield JD. 2010. MCMC methods for multiresponse generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. (http://www.jstatsoft.org/v33/i02)20808728 [Google Scholar]
- 15.Jetz W, Thomas G, Joy J, Hartmann K, Mooers A. 2012. The global diversity of birds in space and time. Nature 491, 444–448. ( 10.1038/nature11631) [DOI] [PubMed] [Google Scholar]
- 16.Bennett PM, Owens IPF. 1997. Variation in extinction risk among birds: chance or evolutionary predisposition? Proc. R. Soc. Lond. B 264, 401–408. ( 10.1098/rspb.1997.0057) [DOI] [Google Scholar]
- 17.Starrfelt J, Kokko H. 2012. Bet-hedging—a triple trade-off between means, variances and correlations. Biol. Rev. 87, 742–755. ( 10.1111/j.1469-185X.2012.00225.x) [DOI] [PubMed] [Google Scholar]
- 18.Anderson M, Åhlund M. 2012. Don't put all your eggs in one nest: spread them and cut time at risk. Am. Nat. 180, 354–363. ( 10.1086/667191) [DOI] [PubMed] [Google Scholar]
- 19.Davies NB, Brooke M de L. 1988. Cuckoos versus reed warblers: adaptations and counteradaptations. Anim. Behav. 36, 262–284. ( 10.1016/S0003-3472(88)80269-0) [DOI] [Google Scholar]
- 20.Rothstein SI. 1990. A model system for coevolution: avian brood parasitism. Annu. Rev. Ecol. Evol. 21, 481–508. ( 10.1146/annurev.es.21.110190.002405) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


