Table 2. Ionization Constants of New Dibasic Antitrypanosomal Compounds Determined by the 96-Well UV Spectrophotometric Method at 30 °C and Constant Ionic Strength (I = 0.1 M).
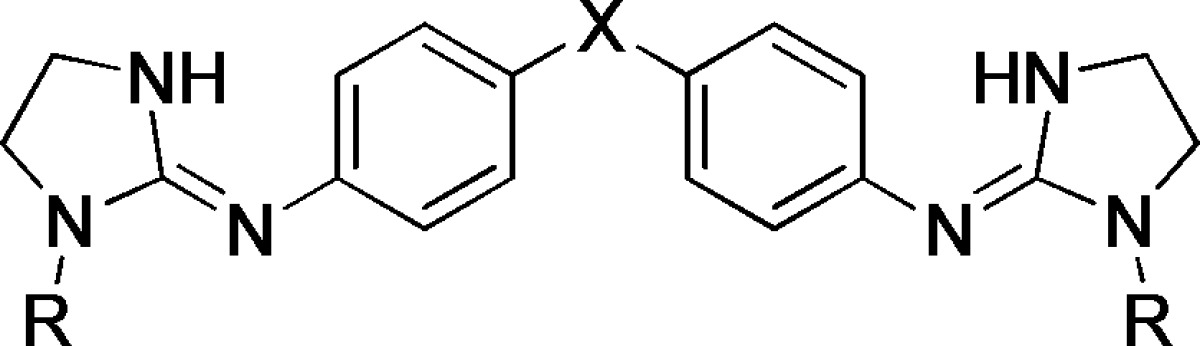
| compda | X | R | λ (nm)b | pKac |
|---|---|---|---|---|
| 6a | NHCO | H | 258/308 | 9.29 ± 0.07d |
| 6b | OH | 258/298 | 7.43 ± 0.10 | |
| 6c | OMe | 258/312 | 7.27 ± 0.09 | |
| 6d | OEt | 258/308 | 7.34 ± 0.03 | |
| 7a | CH2CH2 | H | 260/310 | 10.71 ± 0.10 |
| 7b | OH | 238/264 | 7.97 ± 0.13 | |
| 7c | OMe | 236/262 | 8.01 ± 0.12 | |
| 7d | OEt | 238/262 | 8.01 ± 0.21 | |
| 8a | NHCONH | H | 260/292 | 10.34 ± 0.04 |
| 8c | OMe | 240/270 | 7.95 ± 0.05 | |
| 8d | OEt | 258/296 | 8.27 ± 0.07 |
The compounds were dissolved in DMSO (stock solution) and diluted with the corresponding buffers to reach a final concentration of 0.2 mM in the well (the final quantity of DMSO is 2% v/v).
Analytical wavelength.
pKa of the aminoimidazoline groups; only one pKa could be calculated for both imidazolines.
The pKa values are the mean of three or four (for 7c and 8d) independent measurements ± SDs.
