Figure 1.
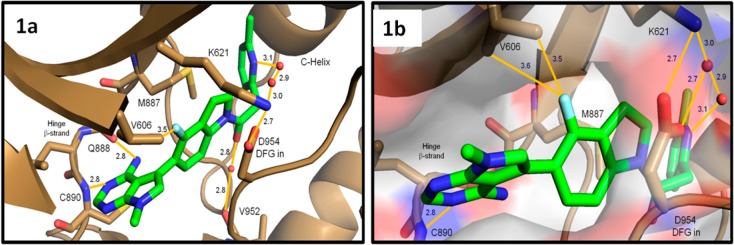
Crystal structure of compound 6 bound to the PERK kinase domain. (a) Close-up view of 6 (green sticks) in the PERK active site (brown cartoon and sticks). The 6-methylpyridyl group occupies the large inner lipophilic pocket, while the fluoroindoline fills a narrow channel between M887 and D954 of the inward facing DFG motif. The amino-pyrimidine binds the hinge β-strand with hydrogen bonds to the backbone amide atoms of Q888 and C890. (b) Close-up view of 6 bound to the PERK active site with surface rendering. Close spatial arrangement between the indoline fluorine atom and the V606 methyl groups may account for some of the improved biochemical activity. Atomic interactions are indicated with orange solid lines and distances reported in Angstroms. The crystal structure of compound 6 bound to the PERK kinase domain is available with the PDB access code 4M7I .
