| Title: | Dihydropyridone P1 as Factor XIa Inhibitors | ||
| Patent Application Number: | WO 2014/022766 Al | Publication date: | 6 February 2014 |
| Priority Application: | US 61/679,197 | Priority date: | 3 August 2012 |
| US 61/786,992 | 15 March 2013 | ||
| Inventors: | Yang, W.; Corte, J. R.; Gilligan, P. J.; Pinto, D. J. P. P.; Ewing, W. R.; Wang, Y. | ||
| Assignee Company: | Bristol-Myers Squibb Company, Route 206 and Province Line Road, Princeton, NJ 08543-4000, USA | ||
| Disease Area: | Thromboembolic disorders, retinal vascular permeability associated with diabetic retinopathy, and diabetic macular edema. | Biological Target: | Inhibition of coagulation factor XIa (FXIa) and/or plasma kallikrein |
| Summary: | The invention in this patent application relates to macrocyclic compounds represented generally by formula (I) which are inhibitors of factor XIa and/or plasma kallikrein. These compounds may potentially provide treatments for thromboembolic disorders and/or retinal vascular permeability associated with diabetic retinopathy and diabetic macular edema. | ||
| In spite of the availability of many anticoagulants and antiplatelet agents, thromboembolic diseases remain a leading cause of death in developed countries. Warfarin, which inhibits the post-translational maturation of coagulation factors VII, IX, and X and prothrombin, is one of the most prescribed anticoagulants. However, it displays a narrow therapeutic index, slow onset of therapeutic effect, numerous dietary and drug interactions, and a need for monitoring and dose adjustment. There is thus a need to discover new safe and effective oral anticoagulants for the prevention and treatment of thromboembolic disorders. | |||
| Coagulation factor XIa (FXIa) is a plasma serine protease that is involved in the regulation of blood coagulation and plays a key role in propagating the amplification loop process of coagulation. The inhibition of the coagulation factor XIa is thus an attractive target for antithrombotic therapy to potentially provide such needed safe and effective treatment of thromboembolic disorders. | |||
| Plasma kallikrein is a serine protease with an amino acid sequence that shares about 58% homology with that of factor XI. It is believed to play a role in a number of inflammatory disorders such as hereditary angioedema (HAE). Plasma kallikrein cleaves high molecular weight kininogen to form bradykinin, which leads to increased vascular permeability. Large protein inhibitors of plasma kallikrein have been shown to be effective in the treatment of HAE by preventing the release of bradykinin. Recent studies on diabetic rats have implicated plasma kallikrein in retinal vascular dysfunctions. It has also been associated with other diabetes complications such as cerebral hemorrhage, nephropathy, cardiomyopathy, and neuropathy. Therefore, inhibition of plasma kallikrein is a viable therapeutic target for the treatment of these disorders. | |||
| The use of large protein plasma kallikrein inhibitors is associated with the risk of anaphylactic reactions, and currently, there are no approved synthetic small molecule plasma kallikrein inhibitors. Known small molecule inhibitors of plasma kallikrein contain highly polar and ionizable guanidine or amidine functionalities that may limit their gut permeability and oral bioavailability. Thus, there is a need for new orally bioavailable small molecule inhibitors of plasma kallikrein that do not induce anaphylaxis. | |||
| Important Compound Classes: | 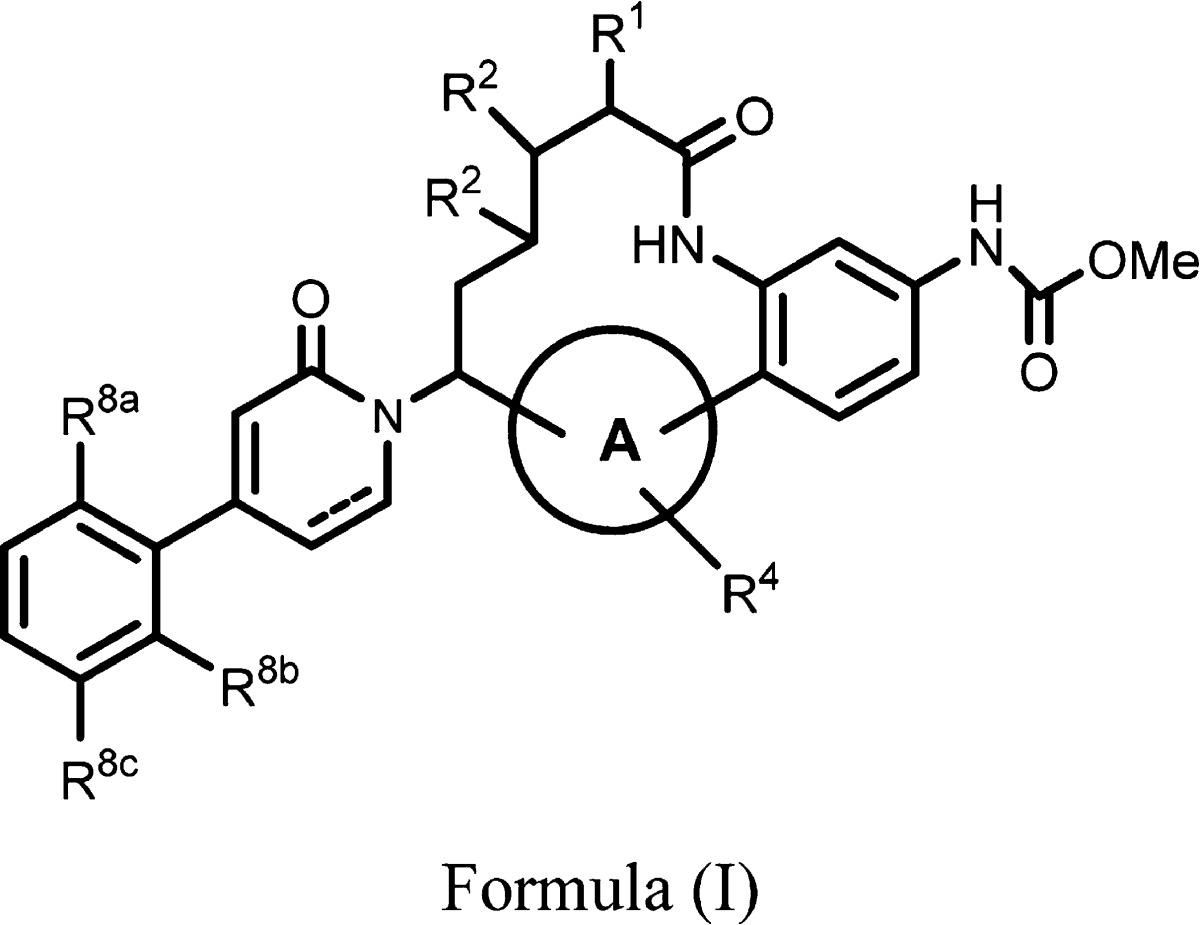 |
||
| Key Structures: | The inventors reported the structures of 29 specific examples of formula (I) including the following four representative compounds: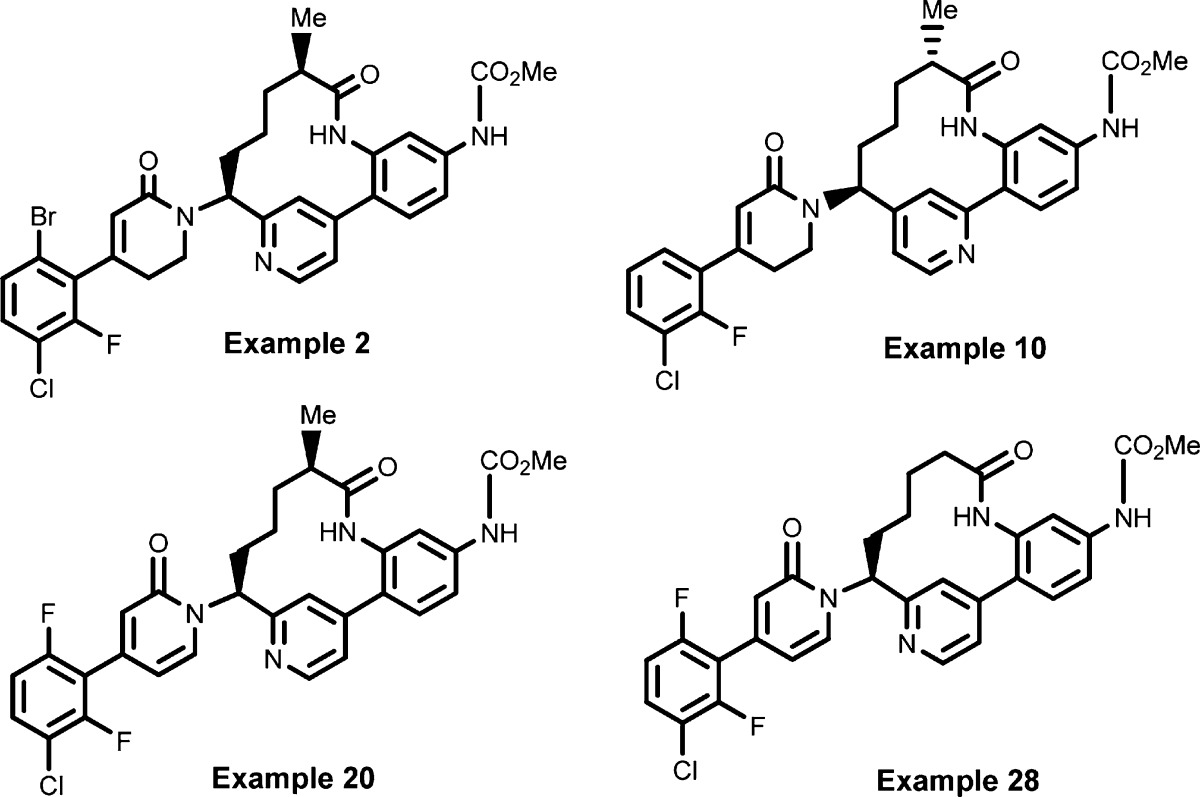
|
||
| Biological Assay: | In Vitro Assays | ||
| Testing the effectiveness of the compounds as inhibitors of the coagulation Factors XIa, VIla, IXa, Xa, and XIIa, plasma kallikrein, or thrombin. | |||
| In Vivo Assays | |||
| a. In Vivo Electrically Induced Carotid Artery Thrombosis (ECAT) Model | |||
| b. In Vivo Rabbit Arterio-venous (A V) Shunt Thrombosis Model | |||
| Biological Data: | The inventors reported data from the in vitro assays showing the inhibitory activity of Factor XIa and Plasma Kallikrein (Ki values) for all 29 examples. The data for selected examples (structures above) are listed in the following table: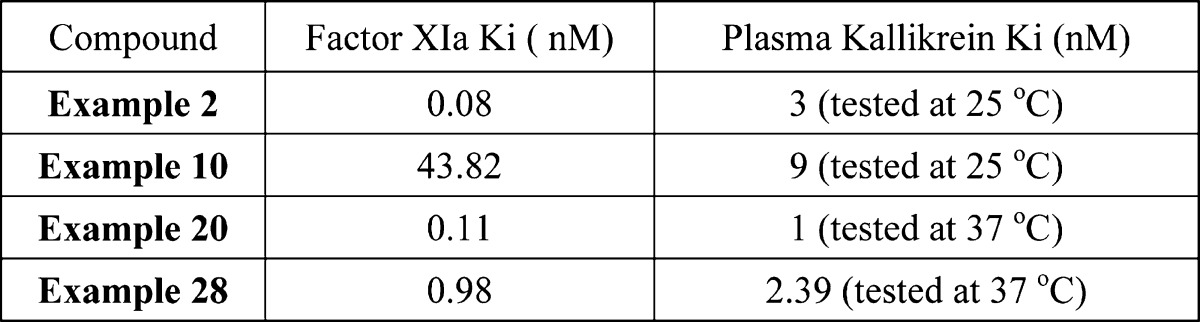
|
||
| Recent Review Articles: | He R.; He S.. Xueshuan Yu Zhixuexue (Chin. J. Thrombosis Hemostasis) 2011, 17 (6), 243–246. | ||
| Schumacher W. A.; Luettgen J. M.; Quan M. L.; Seiffert D. A.. Arterioscler. Thromb. Vasc. Biol. 2010, 30 (3), 388–392. | |||
| Feener E. P.; Zhou Q.; Fickweiler W.. Thromb. Hemostasis 2013, 110 (3), 434–441. | |||
| Bjoerkqvist J.; Jaemsae A.; Renne T.. Thromb. Hemostasis 2013, 110 (3), 39–407. | |||

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.