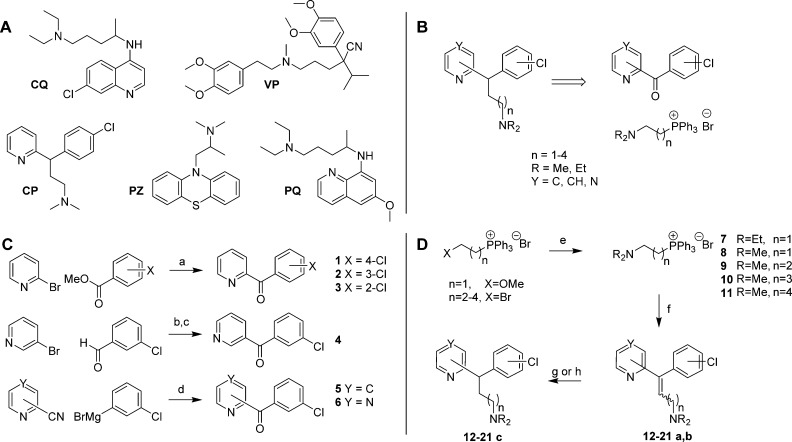
Figure 1.
Chemical structures and synthesis. (A) The structures of chloroquine (CQ), verapamil (VP), chlorpheniramine (CP), promethazine (PZ), and primaquine (PQ). (B) Retrosynthesis of CP analogues. (C) Synthesis of ketones 1–6. Reagents and conditions: (a) n-BuLi, THF, ether, −85 °C warming to −10 °C, 15 min, then −110 °C warming to r.t., 24 h (50–77%); (b) n-BuLi, ether, −78 °C, 1 h warming to r.t., 1.5 h (91%); (c) MnO2, 11 days (78%); (d) ether, toluene, 80 °C, 1.75 h then 2 M H2SO4, 0.5 h (64–66%). (D) Synthesis of CP analogues 12–21c. Reagents and conditions: (e) R2NH(aq), MeOH, 80 °C, 18 h (96–99%); (f) LiN(SiMe3)2, THF, 0 °C, 15 min, then 1–6, r.t., 24 h (33–83%); (g) Pd/C, H2, EtOH, 6.5–13 h (46–50%); (h) PtO2, H2, EtOH, 3.5–6.5 h (77–99%).