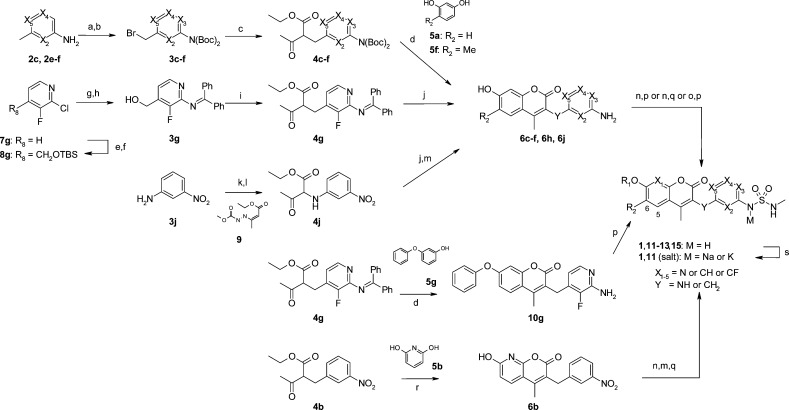
Scheme 2. Synthesis of Coumarins 1, 11–13, and 15.
Reagents and conditions: (a) (Boc)2O, 60 °C, then (Boc)2O, THF, DMAP, rt; (b) NBS, benzoylperoxide or AIBN, CCl4, reflux; (c) ethyl acetoacetate, NaH, THF, 0 °C; (d) 5, conc. H2SO4; (e) LDA, THF, DMF, −78 to 0 °C, then NaBH4, 0 °C, 58%; (f) TBSCl, imidazole, THF, rt, 93%; (g) benzophenone imine, NaOtBu, Pd2(dba)3, rac-BINAP, toluene, 60 °C; (h) TBAF, THF, 76% (2 steps); (i) MsCl, LiOtBu, THF, 0 °C, then ethyl acetoacetate, LiOtBu, NaI, THF, 50 °C, 3 h, 95%; (j) 5a, MsOH, CF3CH2OH; (k) 9, THF, 70 °C, 12 h, 78%; (l) TiCl3, 20–30% HCl aq., acetone, rt, 30 min, 71%; (m) SnCl2·2H2O, EtOAc, 75 °C; (n) R1X, Cs2CO3 or K2CO3 or NaH, DMF; (o) R1X, CuI, N,N’-dimethylethylenediamine, Cs2CO3, DMF, 100 °C; (p) N-methylsulfamoyl chloride, pyridine, DMF; (q) N-methyl-2-oxooxazolidine-3-sulfonamide, Et3N, MeCN, 80 °C; (r) 5b, Zn(OTf)2, MeOH, reflux; (s) NaOH or KOH, MeOH.