Table 2. Antiproliferative Activity of Biphenyl Derivatives with Various Phenyl Substitutions.
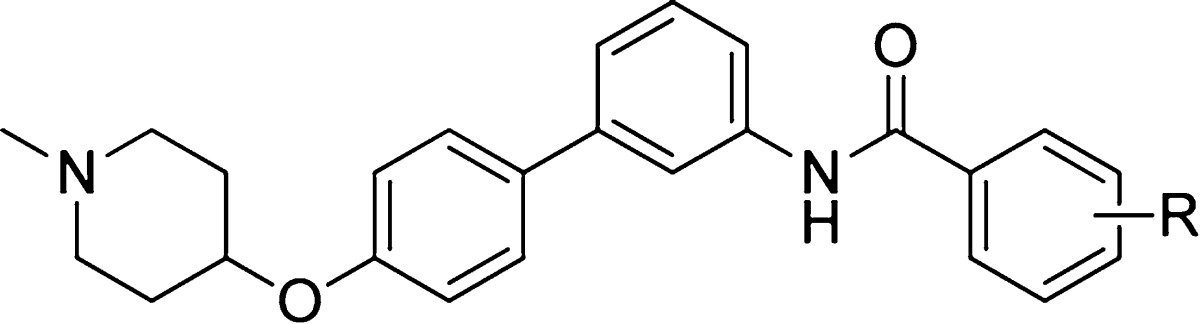
| Entry | R | SKBr3 (IC50, μM) | MCF-7 (IC50, μM) |
|---|---|---|---|
| 11a | H | 18.86 ± 0.95 | 12.02 ± 0.57 |
| 11b | p-CH3 | 5.27 ± 0.29a | 3.92 ± 0.13 |
| 11c | m-CH3 | 11.38 ± 1.37 | 7.73 ± 1.90 |
| 11d | p-t-butyl | 1.51 ± 0.31 | 3.45 ± 0.02 |
| 11e | p-methoxy | 10.1 ± 0.93 | 5.52 ± 0.01 |
| 11f | m-methoxy | 8.36 ± 1.35 | 4.50 ± 0.46 |
| 11g | p-Cl | 3.63 ± 1.03 | 2.23 ± 0.05 |
| 11h | m-Cl | 4.29 ± 0.43 | 2.11 ± 0.42 |
| 11i | o-Cl | 7.87 ± 0.48 | 5.17 ± 0.49 |
| 11j | p-Br | 1.94 ± 0.11 | 0.88 ± 0.07 |
| 11k | 3,4-dichloro | 2.24 ± 0.11 | 2.17 ± 0.37 |
| 11l | 2,4-dichloro | 5.91 ± 0.15 | 3.93 ± 0.47 |
| 11m | 3,5-dichloro | 4.23 ± 0.09 | 3.72 ± 0.15 |
| 11n | (2-naphthoyl) | 2.09 ± 0.34 | 1.66 ± 0.27 |
| 11o | (1-naphthoyl) | 1.64 ± 0.13 | 1.10 ± 0.17 |
Values represent mean ± standard deviation for at least two separate experiments performed in triplicate.
