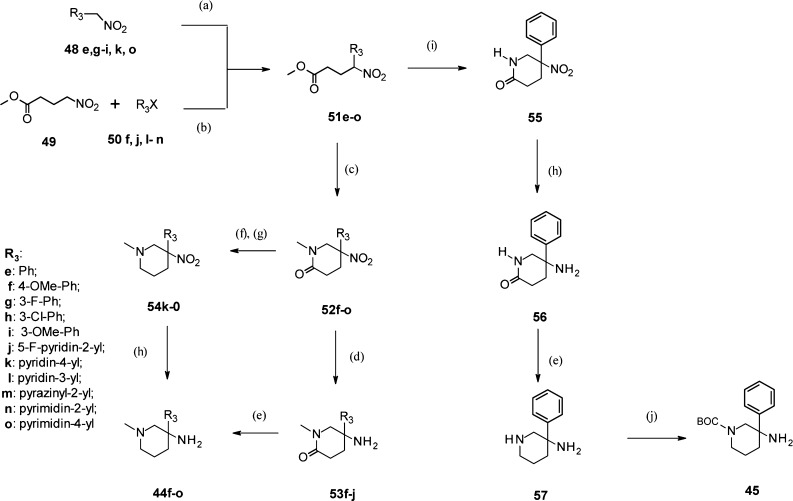
Scheme 3. Synthesis of N-Methyl-3-amino-piperidines 44f–o and N-Boc-3-amino-3-phenyl-piperidine 45.
Reagents and conditions: (a) methyl acrylate, amberlyst A-21, dioxane, RT, 41–85%; (b) cat. Pd2dba3, cat. di-tert-butyl-(2′-methyl-biphenyl-2-yl)-phosphane, Cs2CO3, DME, reflux, 10–90%; (c) MeNH2 (41% in water), CH2O (36% in water), dioxane, RT then 65 °C, 49–94%; (d) zinc, HCl, dioxane, RT, 13–86%; (e) LiAlH4, THF, RT, 71–86%; (f) Lawesson’s reagent, toluene, 80 °C, 77–100%; (g) NaBH4, MeOH, RT, 49–76%; (h) Ra–Ni, H2 (1 atm.), THF, 0 °C, 87–100%; (i) NH4OAc, CH2O (36% in water), EtOH, reflux, 84%; (j) Boc2O, Et3N, CH2Cl2, RT, 70%.