Figure 1.
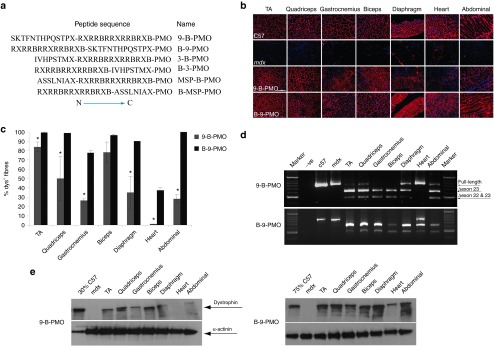
Systemic administration of 9-B-PMO and B-9-PMO conjugates in adult mdx mice. Dystrophin expression following single 25 mg/kg intravenous injections of the 9-B-PMO and B-9-PMO conjugates in young adult mdx mice. (a) Schematic figure illustrating the six different AO constructs utilized. PMO contains the sequence of GGCCAAACCTCGGCTTACCTGAAAT (5′–3′). Peptides are written from N to C orientation using the standard one letter amino acid code except for X and B, which are un-natural amino acids (X, 6-aminohexanoic acid, B, β-alanine). (b) Immunostaining of muscle tissue cross-sections to detect dystrophin protein expression and localization in C57BL/6 normal control (top panel), untreated mdx mice (second panel), 9-B-PMO treated (third panel) and B-9-PMO treated mdx mice (bottom panel). Muscle tissues analyzed were from tibialis anterior (TA), gastrocnemius, quadriceps, biceps, diaphragm, heart, and abdominal wall (abdominal) muscles (scale bar: 200 μm). (c) Quantification of dystrophin-positive fibers in muscle cross-sections from mdx mice treated with 25 mg/kg 9-B-PMO and B-9-PMO. The data is presented as mean ± SEM and significant difference was observed in B-9-PMO treated mdx mice compared with 9-B-PMO (t-test, *P < 0.05; n = 4, n represents the number of biological replicates). (d) RT-PCR to detect exon-skipping efficiency at the RNA level. Exon-skipping products are shown by shorter exon-skipped bands (indicated by Δexon23 – exon 23 deleted; Δexon22&23 – both exon 22 and 23 skipped). (e) Western blot for dystrophin expression in 9-B-PMO and B-9-PMO treated mdx mice. Equal loading of 10-µg protein is shown for each sample except for the C57BL/6 control lanes where 5 and 2.5 µg protein was loaded, respectively. α-actinin was used as loading control. There was no visible difference in the size of dystrophins between muscle treated with peptide-PMO conjugates and muscle from the normal C57BL/6 mouse. AO, antisense oligonucleotides; MSP, muscle-targeting peptide; PMO, phosphorodiamidate oligomers.
