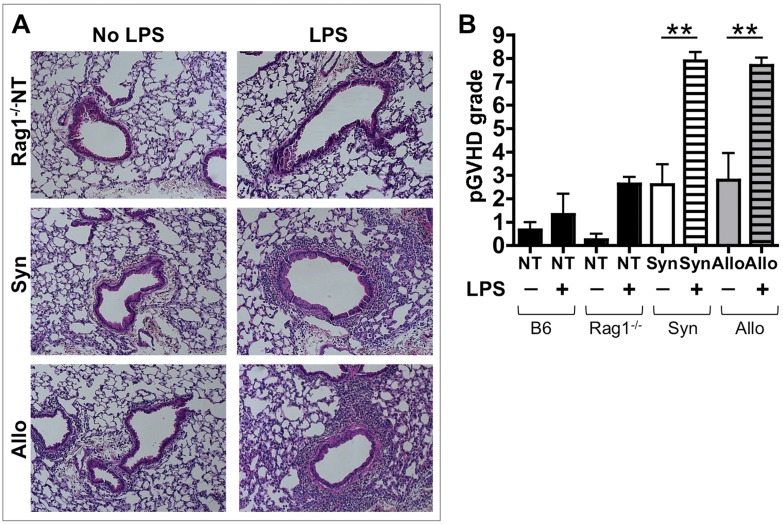
Figure 1. Splenocyte transfer followed by inhaled LPS leads to pGVHD pathology.
Rag1−/− mice received allogeneic (Allo) or syngeneic (Syn) splenocytes or no splenocyte transfer (Rag1−/−NT). Additional wild-type C57BL/6 (B6) mice without splenocyte transfer (B6NT) were used as controls. Allo, Syn, Rag1−/−NT and B6NT mice underwent daily exposures to aerosolized LPS for 5 days starting 1 week after splenocyte transfer. Mice were euthanized 72 hours after the last LPS exposure. (A) Lung pathology assessment shows perivascular and peribronchiolar mononuclear inflammation in the AlloLPS and SynLPS mice (H&E, 100X). Only minimal inflammation is seen in AlloNoLPS and SynNoLPS lungs. Rag1−/−NT mouse lung pathology is shown for additional comparison and is similar to that of B6NT mouse lungs. After LPS, all NT mice have rare mononuclear cells visible in the perivascular and peribronchiolar structures. This is similar to pathology seen in B6NT mice. (B) Lung pGVHD pathology was graded in a blinded fashion using a 0–9 semi-quantitative grading schema to express the thickness of the mononuclear infiltrate around airways and around vessels as well as the overall extent of the pathology in the lung. SynLPS and AlloLPS lungs have a grade of about 8, which is significantly higher than the grade of non-LPS exposed controls where the grade is about 2.5 (AlloLPS vs. AlloNoLPS p = 0.003 and SynLPS vs. SynNoLPS p = 0.0005). As measured by this grading, LPS led to low-grade background inflammation in NT mice as shown in the graph. Data represent the average +/− SEM and **represents p<0.005. Data have been replicated in 3 independent experiments.