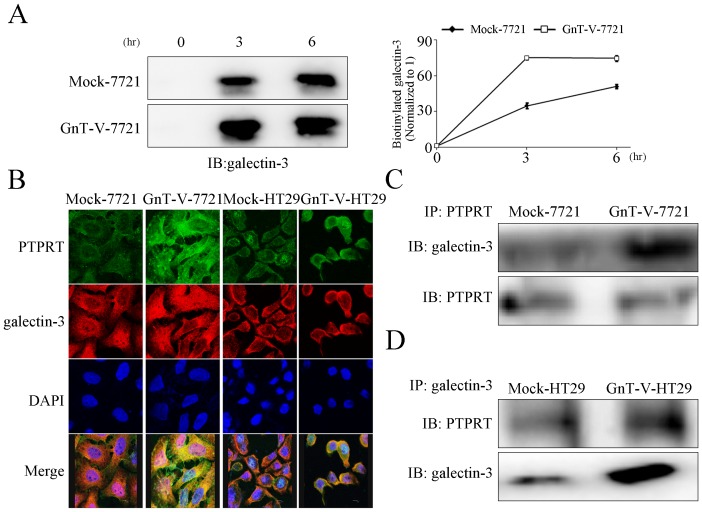
Figure 4. The glycosylation of PTPRT by GnT-V promotes galectin-3 binding, resulting in dimerized PTPRT with increased cell-surface retention.
(A) Cell-surface retention of galectin-3 was examined after cell-surface biotinylation. The stable transfectants were re-cultured for 3 and 6 hours before lysis and streptavidin bounded agarose precipitation. After that galectin-3 was detected by immunoblot using anti-galectin-3 antibody. The graph (right panel) is the quantification of the band intensity. The relative amount of galectin-3 at 3 or 6 hour time point was normalized to that at 0 h in both Mock-7721 and GnT-V-7721. The data represent the mean ± SEM of three independent analyses. (B) More colocalization of galectin-3 with PTPRT was visualized at the cell surface in GnT-V overexpressing cells. Confocal microscopy was taken to detect the localization of galectin-3 and PTPRT in Mock-7721, GnT-V-7721, Mock-HT29, GnT-V-HT29. Mouse anti-PTPRT and rabbit anti-galectin-3 were used as primary antibodies. Cy3-conjugated donkey anti-rabbit IgG and FITC-conjugated goat anti-mouse IgG were secondary antibodies. Cell nuclei were visualized by DAPI staining. Merged panels show the overlapped channels. (C) Increased association of galectin-3 with PTPRT is observed in GnT-V overexpressing cells. Mock-7721 and GnT-V-7721 cells were cross-linked with 3 mM BS3 at 4°C for 2 hours before immunoprcipitation (IP). IP was performed using anti-PTPRT antibody followed by immunoblot with anti-PTPRT and anti-galectin-3 antibodies. (D) Mock-HT29 and GnT-V-HT29 cells were treated as described in Fig. 4C, except for that anti-galectin-3 was used for IP.