Abstract
PURPOSE
Variable airway function is a central feature of the asthmatic condition. Thus, habitually active asthmatics are certain to exercise under conditions of variable airway (dys)function. The purpose of this study was to determine the effects of variable pre-exercise airway function on ventilation during whole-body exercise in asthmatic adults.
METHODS
Eight mild asthmatic (age, 26 yrs; V̇O2peak, 49 ml·kg−1·min−1) and nine non-asthmatic (age, 30 yrs; V̇O2peak, 46 ml·kg−1·min−1) adults performed constant workrate cycling exercise-to-exhaustion following four separate interventions: 1) a control trial (CON); 2) inhalation of fast-acting β2-agonist (BD); 3) eucapnic voluntary hyperpnea challenge (BC); and 4) sham to the hyperpnea (SHAM). Pulmonary function was assessed at baseline and following each intervention. Exercise ventilation and operating lung volumes were compared among the four exercise trials in both control and asthmatic subjects.
RESULTS
Baseline pulmonary function was significantly lower in asthmatic subjects compared with control subjects. In asthmatic subjects, post-intervention (i.e., pre-exercise) forced expiratory volume 1.0 second was significantly different among the four exercise trials (CON, 3.5 ± 0.4; BD, 4.1 ± 0.4; SHAM, 3.6 ± 0.3; BC, 2.8 ± 0.3 L; p < 0.05), whereas it was not different in control subjects. There were no differences in exercise ventilation or operating lung volumes during exercise among the four trials either within asthmatic subjects or between control and asthmatic subjects.
CONCLUSION
These findings suggest that the state of airway function – whether bronchodilated or bronchoconstricted – prior to exercise in the mild asthmatic does not affect the exercise ventilatory response. Ventilatory system function in the asthmatic thus appears to be responsive to the acute requirement for increased airflow during whole-body exercise.
Keywords: Airway caliber, airway mechanics, bronchoconstriction, bronchodilation, exercise-induced bronchodilation, pulmonary mechanics
INTRODUCTION
Variable airway function is a central feature of the asthmatic condition. Asthmatics thus exhibit daily and seasonal variation in airway inflammation, airways hyperresponsiveness (AHR), pulmonary function, and clinical symptoms (8, 14, 17). Exposure to a variety of immunological triggers such as pollens, pet dander, mold, and dustmites instigate airway inflammation, airway smooth muscle contraction, and worsened airway function. Several other non-immunological triggers exist as well, including inhalation of cold/dry air, exposure to environmental pollutants, and whole-body exercise. Importantly, AHR demonstrates seasonal variability and fluctuates with variations in environmental allergen concentrations, and in some asthmatics AHR regularly varies over the course of 24 hours (8, 20). For all of these reasons, habitually active asthmatics are certain to exercise under conditions of variable airway (dys)function. The influences of this variable airway function on the responses to whole-body exercise are not known.
Regular exercise is a generally recommended therapeutic modality in patients with asthma (23, 32, 34). These recommendations are partially informed by studies demonstrating improved aerobic capacity with training and unchanged pulmonary function and exercise-induced bronchoconstriction (EIB) over time with training; pulmonary function and EIB neither worsen nor improve with training (34, 40). Very few studies, however, have examined relationships between baseline airway function and exercise ventilation in the asthmatic. This is an important gap in the literature, as the variable airway function in asthma may interact with the exercise ventilatory response. This contrasts sharply with non-asthmatic persons, in which airway function is consistent on a day-to-day basis. Furthermore, the majority of studies assessing responses to exercise in asthmatic subjects have not included comparisons with non-asthmatic control subjects. In general, the most meaningful insight into the physiological responses to exercise in a patient population can only be gleaned through comparisons with non-diseased control subjects. Improved understanding of the relationships between baseline airway function and exercise ventilation in the asthmatic (and compared with non-asthmatic counterparts) will help to inform exercise recommendations for this large clinical population.
It is reasonable to postulate a relationship between baseline airway function and exercise ventilation in the asthmatic. For example, pre-exercise bronchodilation might be expected to allow for increased exercise ventilation in the asthmatic. However, studies have consistently demonstrated an unchanged exercise ventilation following bronchodilation in asthmatic subjects (11, 18, 35, 36). Conversely, a small number of investigations have experimentally worsened airway function prior to exercise in asthmatic subjects. In one experimental approach, exercise is preceded by induction of airway narrowing via osmotic stress, including inhalation of hypertonic saline (5) or voluntary eucapnic hyperpnea (6), or inhalation of the bronchoconstrictor histamine (13). These studies, however, were not designed to carefully assess the effects of pre-exercise bronchoconstriction on ventilation during exercise, focusing instead on post-exercise airway function in the asthmatic. Thus, the role of pre-exercise bronchoconstriction on ventilation during exercise in the asthmatic remains unknown.
The purpose of this study was to determine the effects of both improved and worsened pre-exercise airway mechanical function on the ventilatory responses to whole-body exercise in asthmatic adults as well as healthy controls. We hypothesized that exercise ventilation would be similar despite variable pre-exercise airway function in asthmatic subjects.
METHODS
Subject selection
Adult male and female asthmatic and non-asthmatic subjects were recruited through advertisements in local newspapers and by flyers posted on the Johnson State College campus and within the local community. Prior to participation, subjects were fully informed of the procedures, risks, and benefits of the study, and written, informed consent was obtained. The experiments described in this proposal were approved by the Johnson State College institutional review board for research involving human subjects.
All participants were between the ages of 18-45 y, had a negative history for cardiovascular disease and other chronic illness (excepting asthma), were non-smokers, and had an absence of respiratory infection during the 6-weeks prior to participation. Asthmatic subjects were limited to those with mild disease that was controlled or partly controlled, as defined by the Global Initiative for Asthma (12). Asthmatic subjects currently taking inhaled or oral steroids were excluded from participation, as were those who had attended the emergency room for an asthma exacerbation during the previous 3 years. All subjects completed two separate screening studies on different days to determine eligibility for participation as an asthmatic or non-asthmatic subject.
Screening studies
Exercise-induced bronchospasm
All subjects completed an incremental exercise test-to-exhaustion. The inspirate consisted of dry air from a compressed gas tank (FIO2, .21, balance nitrogen). Pulmonary function was assessed prior to exercise, and at 5, 10, 15, 20, and 30 minutes following exercise.
Bronchodilator reversibility
Following baseline pulmonary function tests (PFT), subjects inhaled 4 actuations of a fast-acting β2-agonist. For each actuation, subjects exhaled to residual volume, depressed the actuator, inhaled slowly through a chamber (aerovent) to total lung capacity, and held their breath for 3-5 seconds prior to exhaling. Pulmonary function was assessed at several time-points following β2-agonist inhalation.
Participants meeting at least one of the following two criteria were classified as asthmatic subjects: 1) ≥ 12% decrease in forced expiratory volume 1.0 second (FEV1.0) after the incremental exercise test-to-exhaustion; 2) ≥ 12% increase in FEV1.0 after inhalation of the fast-acting β2-agonist.
Experimental design
All subjects completed four separate exercise studies with four different pre-exercise interventions. The interventions included: 1) four actuations of a fast-acting β2-agonist to improve airway function; 2) a eucapnic voluntary hyperpnea challenge to worsen airway function; 3) a sham to the hyperpnea; and 4) a control trial. The order of experimental interventions for each subject was randomized, and intervention order was balanced among subjects within the control and asthmatic group. Following each intervention, subjects completed an exercise bout on a magnetized cycle ergometer. Subjects were instructed to refrain from ingesting caffeine for eight hours prior to each study and inhaled β2-agonist for twelve hours prior to each study.
Experimental interventions
All subjects completed four separate exercise challenges preceded by a different intervention, as follows.
β2-agonist [bronchodilation (BD)]
Following baseline (BL) PFT’s, subjects inhaled four actuations of a fast-acting β2-agonist. Pulmonary function was assessed at 5 and 10 minutes following inhalation [post-intervention, (PI)]. Exercise was commenced at 15 minutes following inhalation, corresponding closely with the time of maximal bronchodilation.
Eucapnic voluntary hyperpnea [bronchoconstriction (BC)]
The eucapnic voluntary hyperpnea challenge (EVH) was completed in accordance with the methods described by Anderson and Brannan (2). Briefly, subjects ventilated dry, compressed gas consisting of 21% O2 and 5% CO2 at a ventilation rate equal to FEV1.0 × 30. Pulmonary function was performed at 5 and 10 minutes following the challenge. Exercise was commenced 15 minutes following EVH, closely corresponding with the time of maximal airway narrowing.
Hyperpnea sham (SHAM)
A sham trial was used to control for subject expectation effects or behavioral responses to the EVH protocol. Utilizing the same apparatus used for the EVH challenge, subjects were instructed to breathe in a normal, relaxed manner. Subjects inhaled room air during this trial; however, they were told that they were breathing air from the tank of compressed gas. The Douglas bag was filled with gas prior to the study, and laboratory personnel mimicked adjustment of a three-way valve and briefly opened the nozzle on the tank occasionally during the experiment. Pulmonary function was assessed at 5 and 10 minutes after the protocol, after which time exercise was commenced.
Control (CON)
Following baseline PFT’s, subjects rested in a comfortable, seated position for 10 minutes. Pulmonary function was re-assessed and followed immediately by the exercise test.
Pulmonary function
PFT’s were measured with a Medgraphics automated spirometer. All pulmonary function tests were completed in the seated, upright position according to recommendations by the American Thoracic Society and European Respiratory Society (29). During each measurement, subjects completed maximal volitional flow-volume maneuvers for determination of peak expiratory flow, forced vital capacity (FVC), forced expiratory volume 1.0 second (FEV1.0), and forced expiratory flow at 50% of FVC (FEF50%). Slow vital capacities were performed for determination of inspiratory capacity (IC). Predicted values are from Quanjer (33).
Exercise apparatus
All exercise was completed on a magnetically braked cycle ergometer (Velotron). Subjects breathed through a two-way, non-rebreathing valve (Hans-Rudolph) with noseclips in place. Compressed gas (21% O2, balance nitrogen) was used as the inspirate for all exercise studies in both asthmatic and non-asthmatic subjects. Separate pneumotachographs (Hans Rudolph) were used to determine inspiratory and expiratory flowrates. Separate oxygen and carbon dioxide gas analyzers (AEI Technologies) were used to analyze expired gases. A Powerlab 16-channel data acquisition system (ADinstruments) interfaced with a laptop computer was used to collect resting and exercise data.
Exercise protocol
Prior to exercise, subjects performed multiple IC maneuvers and several maximal volitional flow-volume loops. Following resting data collection, subjects completed three minutes of exercise at a workload equal to 70% of the peak workload achieved during the maximal exercise test (WU). Exercise workload was then increased to 85% of peak workload and was continued to volitional exhaustion (prolonged). Inspiratory capacities were performed during WU and at regular intervals during prolonged exercise.
Exercise measurements
Inspired and expired airflow rates and expired gases were used to calculate metabolic oxygen consumption (V̇O2) and carbon dioxide production (V̇CO2), minute ventilation (V̇e), tidal volume (Vt), breathing frequency (fb) and IC. A Polar heart rate monitor (POLAR, USA) was used to record heart rate during exercise. A modified Borg scale (0-10) was used to rate perceived exertion for both breathing and leg discomfort.
Statistical analysis
A three-factor [GROUP (asthma vs. non-asthma) × TRIAL (control, β2-agonist, hyperpnea, sham) × TIME (exercise time)] repeated measures analysis of variance was used to analyze the exercise responses. When significant main effects were found, Bonferroni-corrected multiple pairwise comparisons were employed for post-hoc analysis. Linear regression was used to analyze relationships between selected variables. Tabular data are presented as mean ± standard deviation. Data in figures are presented as mean ± standard error of mean. Significance was set at α < 0.05. The statistical software program SYSTAT was used to analyze all data (SYSTAT 12).
RESULTS
Group characteristics
Group descriptive characteristics and results from the screening studies are shown in Table 1. There were no differences in age, height, weight, V̇O2peak, or exercise peak power output between control and asthmatic subjects. Baseline pulmonary function and the change in FEV1.0 after both bronchodilator and exercise were significantly different between asthmatic and control subjects. Thus, the two groups were well matched for anthropometric characteristics and exercise capacity, but AHR was only present in asthmatic subjects. Of the eight asthmatic subjects, six were prescribed an inhaled β-agonist on an as needed basis.
Table 1.
Descriptive characteristics for control and asthmatic subjects.
| Control Group (n=9) | Asthmatic Group (n=8) | |
|---|---|---|
| Male/Female | 6/3 | 5/3 |
| Age, y | 25.6 ± 8.8 | 30.4 ± 8.4 |
| Height, m | 1.73 ± 0.1 | 1.72 ± 0.1 |
| Weight, kg | 74.2 ± 11.8 | 74.4 ± 13.9 |
| Body mass index (kg·m2) | 24.7 ± 2.6 | 25.1 ± 2.7 |
| VO2peak, ml−1·kg·−1min−1 | 48.5 ± 6.4 (122 ± 0.3) | 46.1 ± 6.6 (121 ± 0.3) |
| FVC, L | 5.29 ± 1.07 (115 ± 13) | 5.38 ± 1.2 (121 ± 17) |
| FEV1.0, L | 4.45 ± .94 (113 ± 11) | 3.77 ± 1.1 (99 ± 17) |
| FEV1.0/FVC | 0.84 ± 0.07 (105 ± 8.4) | 0.70 ± 0.10 (87 ± 13)* |
| FEF50%, L·sec−1 | 5.27 ± 1.56 (102 ± 24) | 3.29 ± 1.3 (65 ± 22)* |
| PEF, L·sec−1 | 9.35 ± 2.28 (104 ± 13) | 7.89 ± 2.2 (91 ± 19) |
| Peak power output, watts | 262 ± 44 | 275 ± 49 |
| BD reversibility, % change FEV10 | + 5.2 ± 4.1 | + 12.2 ± 7.5* |
| EIB, % change FEV10 | −5.3 ± 3.0 | −26.6 ± 15.3* |
V̇O2peak, maximal oxygen uptake; FVC, forced vital capacity; FEV1.0, forced expiratory volume 1 second; FEF50%, forced expiratory flow at 50% FVC; PEF, peak expiratory flow; BD, bronchodilator; EIB, exercise-induced bronchoconstriction.
p<0.05 vs. control group. Percent predicted values in parentheses. Spirometry predicted values from Quanjer (33).
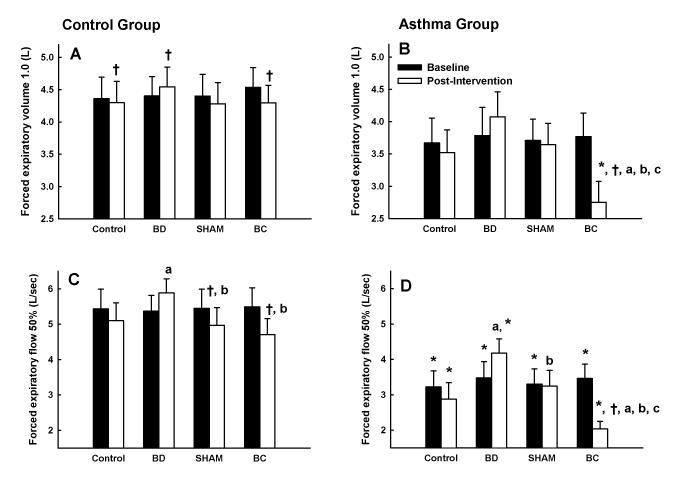
Pulmonary function
Group mean results for FEV1.0 and FEF50% at baseline (i.e., upon arrival to lab) and after each intervention (PI) in control and asthmatic subjects are shown in Figure 1. Overall, FEV1.0 and FEF50% were lower in asthmatic compared with control subjects. In asthmatic subjects, EVH caused a decrease of 1.0 ± 0.6 (−27 ± 15%) and 1.7 ± 0.8 (−43 ± 13%) L, in FEV1.0 and FEF50%, respectively. Moreover, PI values for FEV1.0 and FEF50% were 1.3 ± 0.8 (+57 ± 47%) and 2.3 ± 0.8 (+209 ± 237%) L higher during BD compared with BC in asthmatic subjects. These differences were not seen in control subjects. Results for peak expiratory flow were very similar to FEV1.0 and FEF50% (data not shown). However, there were no significant differences in FVC between the control and asthmatic group or within either group (data not shown). Thus, airway function was markedly different upon exercise commencement among the four experimental trials in asthmatic subjects.
Figure 1.
Forced expiratory volume 1.0 s (FEV1.0) and forced expiratory flow at 50% forced vital capacity (FEF50%) in control and asthmatic subjects at baseline and following an intervention during four experimental trials: control; bronchodilation (BD); sham (SHAM); and bronchoconstriction (BC). A) FEV1.0 at baseline and following each of four interventions in control subjects; B) FEV1.0 at baseline and following each of four interventions in asthmatic subjects; C) FEF50% at baseline and following each of four interventions in control subjects; D) FEF50% at baseline and following each of four interventions in asthmatic subjects. Note that FEV1.0 and FEF50% were lower in asthmatic subjects compared with control subjects. Also, note the marked changes in FEV1.0 and FEF50% following BD and BC in asthmatic subjects. *, p<0.05 vs. control group; †, p<0.05 vs. baseline; a, p<0.05 vs. CON trial; b, p<0.05 vs. BD trial; c, p<0.05 vs. SHAM trial.
Exercise workload & time
All subjects in both groups exercised at 70% (WU) and 85% (prolonged) of their individual peak workload attained during a maximal incremental exercise test. Workload during WU (control group, 177 ± 34 watts; asthma group, 180 ± 39 watts) and prolonged exercise (control group, 220 ± 42 watts; asthma group, 225 ± 46 watts) was not different between control and asthmatic subjects. In control subjects, exercise time-to-exhaustion was 8.0 ± 2.9, 7.4 ± 2.7, 7.7 ± 2.0, and 8.3 ± 2.9 min during CON, BD, SHAM, and BC trials, respectively (p > 0.05). In asthmatic subjects, time-to-exhaustion was 5.6 ± 1.3, 6.0 ± 2.3, 5.4 ± 1.4, and 5.5 ± 2.5 min during CON, BD, SHAM, and BC, respectively (p > 0.05). Group mean exercise time was not different between control and asthmatic subjects among any of the four trials (Bonferroni adjusted p-values: control trial, p=0.177; BD trial, p=1.0; SHAM trial, p=0.214; BC trial, p=0.073).
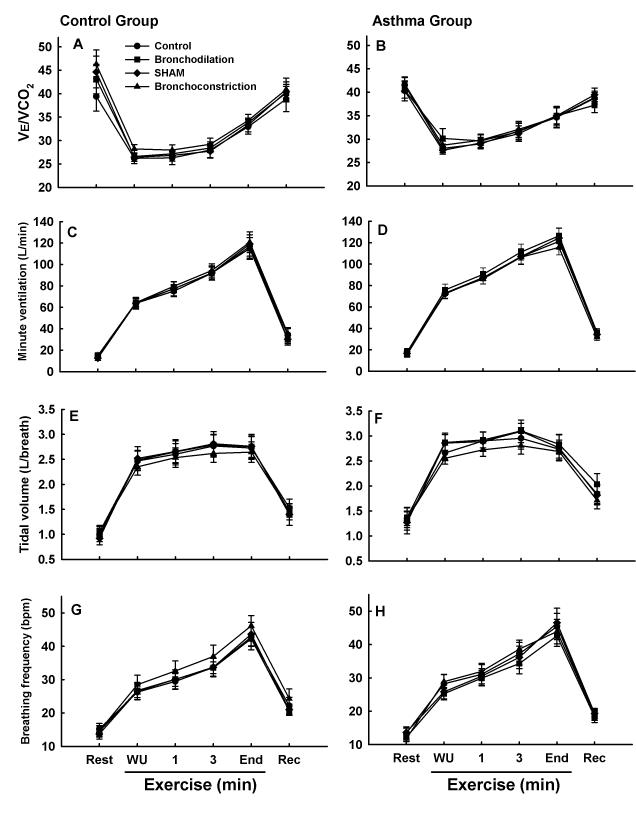
Exercise ventilation
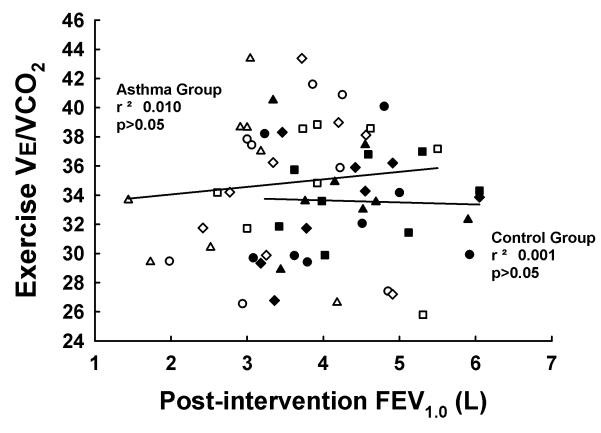
Results for V̇e, Vt, fb, and the ventilatory equivalent for CO2 production (V̇e/V̇CO2) are shown in Figure 2. Within both control and asthmatic subjects, there were no differences among trials for any of the ventilatory variables. Moreover, there were no differences between control and asthmatic subjects for any of the variables among the four experimental trials. Figure 3 depicts the relationship between PI FEV1.0 and exercise V̇e/V̇CO2 in control and asthmatic subjects. In both control and asthmatic subjects, there was no relationship between PI FEV1.0 and exercise V̇e/V̇CO2. These results highlight the dissociation between baseline airway function and exercise ventilation in control and asthmatic subjects.
Figure 2.
Ventilatory variables at rest, during warmup exercise (WU), during exercise-to-exhaustion (1 minute, 3 minutes, end exercise), and three minutes after exercise (Rec) in control and asthmatic subjects during four experimental trials. Control group results are shown in A, C, E, and G. Asthma group results are shown in B, D, F, and H. There were no significant differences for any variable within either the control or asthmatic group. Moreover, there were no significant differences between control and asthmatic subjects for any of the variables.
Figure 3.
Correlations between post-intervention forced expiratory volume 1.0 second (FEV1.0) and exercise ventilatory equivalent for CO2 production (V̇e/V̇CO2) in control (dark symbols) and asthmatic (open symbols) subjects. There was no relationship between post-intervention FEV1.0 and V̇e/V̇CO2 in either control or asthmatic subjects. Circles, control trial; squares, bronchodilation trial; diamonds, sham trial; triangles, bronchoconstriction trial.
Exercise lung volumes
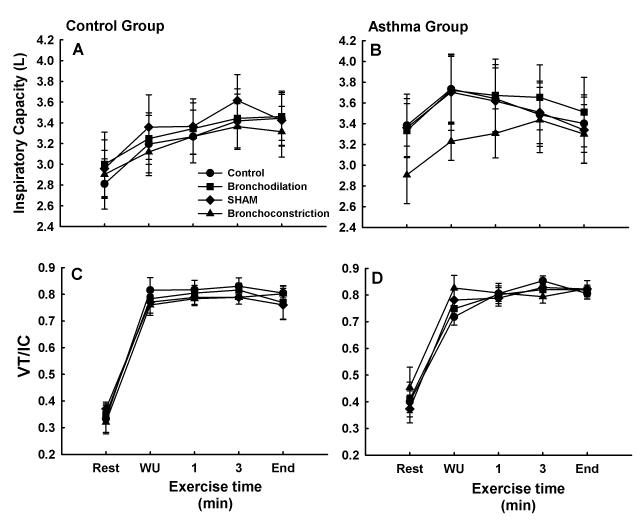
Results for IC and Vt/IC are shown in Figure 4. There were no significant differences in IC or Vt/IC either within or between control and asthmatic subjects. In control subjects, IC exhibited the usual intensity- and time-dependent increase during exercise and exhibited a plateau at the end of exercise. In asthmatic subjects, a significant main effect of exercise trial was found, but bonferroni-corrected pairwise comparisons were not significant. In asthmatic subjects, results for IC were similar during CON, BD, and SHAM, during which IC increased during WU, but thereafter demonstrated a decreasing trend throughout prolonged exercise to approach resting values by end exercise. During BC, IC following intervention was, on average, 0.6 ± 0.3 L lower than the other three trials (p > 0.05). During exercise, however, IC increased so that it equaled the other three trials by the end of exercise.
Figure 4.
Inspiratory capacity (IC) and ratio of tidal volume to inspiratory capacity (Vt/IC) at rest, during warmup exercise (WU), and during exercise-to-exhaustion (1 min, 3 min, end) during four experimental trials in control (A and C) and asthmatic (B and D) subjects. In control subjects, IC and Vt/IC exhibited a similar pattern during all four trials. In asthmatic subjects, note the lower IC at rest and early during exercise during the bronchoconstriction trial compared to the other three trials. Also, note that IC decreased during exercise in asthmatic but not in control subjects. There were no significant differences.
Other exercise responses
In table 2, group mean values for V̇O2, V̇e/V̇O2, HR, RPE-breath, and RPE-leg are shown for control and asthmatic subjects. In general, values for these variables were not different among the four trials within either subject group. Moreover, there were no significant differences between control and asthmatic subjects for any of the variables at any time point among the four exercise trials.
Table 2.
Physiological variables measured at baseline, during warmup exercise, and during exercise-to-exhaustion in control and asthmatic subjects.
| Exercise-to-exhaustion | ||||||
|---|---|---|---|---|---|---|
| Baseline | Warmup | 1 min | 3 min | End | Recovery | |
| Control Group (n=9) | ||||||
|
| ||||||
| VO2, L·min−1 | ||||||
| Control trial | 0.42 ± 0.1 | 2.7 ± 0.6 | 3.1 ± 0.6 | 3.3 ± 0.6 | 3.6 ± 0.9 | 0.67 ± 0.2 |
| BD trial | 0.40 ± 0.1 | 2.6 ± 0.5 | 3.0 ± 0.6 | 3.2 ± 0.6 | 3.4 ± 0.7 | 0.65 ± 0.2 |
| SHAM trial | 0.40 ± 0.1 | 2.6 ± 0.4 | 3.0 ± 0.7 | 3.2 ± 0.7 | 3.5 ± 0.9 | 0.63 ± 0.2 |
| BC trial | 0.40 ± 0.1 | 2.6 ± 0.5 | 3.0 ± 0.7 | 3.2 ± 0.7 | 3.6 ± 0.9 | 0.77 ± 0.3 |
| Ve/VO2 | ||||||
| Control trial | 35.0 ± 16.3 | 24.9 ± 4.8 | 26.1 ± 5.8 | 29.5 ± 6.4 | 34.1 ± 7.7 | 47.3 ± 14.9 |
| BD trial | 36.5 ± 10.0 | 25.2 ± 4.4 | 26.7 ± 4.2 | 29.3 ± 4.9 | 34.3 ± 6.0 | 45.9 ± 8.4 |
| SHAM trial | 36.3 ± 9.4 | 25.3 ± 4.6 | 26.8 ± 5.0 | 29.3 ± 5.0 | 34.5 ± 5.5 | 47.3 ± 8.5 |
| BC trial | 39.6 ± 11.1 | 25.3 ± 3.3 | 27.2 ± 4.3 | 29.9 ± 4.4 | 34.5 ± 5.5 | 45.6 ± 5.5 |
| HR, BPM | ||||||
| Control trial | 71.7 ± 9 | 140.2 ± 14 | 156.9 ± 12 | 167.7 ± 11 | 177.0 ± 8 | 113.1 ± 9 |
| BD trial | 80.4 ± 13 | 144.0 ± 12 | 158.7 ± 12 | 169.1 ± 8 | 177.6 ± 8 | 113.0 ± 11 |
| SHAM trial | 73.8 ± 7 | 144.1 ± 12 | 158.8 ± 12 | 168.6 ± 11 | 178.1 ± 12 | 113.7 ± 17 |
| BC trial | 72.0 ± 6 | 139.8 ± 11 | 157.4 ± 9 | 167.8 ± 9 | 177.4 ± 11 | 111.2 ± 13 |
| RPE, Breath | ||||||
| Control trial | 0.28 ± 0.44 | 1.39 ± 1.02 | 2.78 ± 1.09 | 4.78 ± 1.20 | 7.44 ± 1.59 | 3.33 ± 1.98 |
| BD trial | 0.17 ± 0.35 | 1.17 ± 0.71 | 2.50 ± 1.00 | 4.89 ± 1.27 | 7.67 ± 1.94 | 3.78 ± 1.92 |
| SHAM trial | 0.33 ± 0.71 | 1.56 ± 0.98 | 3.22 ± 0.83 | 5.00 ± 1.12 | 8.33 ± 1.73 | 4.22 ± 2.22 |
| BC trial | 0.22 ± 0.44 | 1.33 ± 0.97 | 3.44 ± 1.01 | 4.89 ± 1.83 | 8.22 ± 1.39 | 3.33 ± 1.66 |
| RPE, Legs | ||||||
| Control trial | 0.17 ± 0.25 | 2.17 ± 1.52 | 4.44 ± 1.67 | 6.00 ± 1.22 | 9.44 ± 0.73 | 5.28 ± 2.41 |
| BD trial | 0.17 ± 0.25 | 2.39 ± 2.00 | 3.78 ± 1.92 | 6.06 ± 1.51 | 8.89 ± 1.05 | 5.00 ± 2.12 |
| SHAM trial | 0.17 ± 0.35 | 2.22 ± 1.37 | 4.11 ± 1.45 | 6.11 ± 1.17 | 9.22 ± 1.09 | 5.33 ± 2.65 |
| BC trial | 0.06 ± 0.17 | 2.56 ± 1.51 | 4.56 ± 0.88 | 6.44 ± 1.33 | 9.22 ± 1.30 | 5.00 ± 2.00 |
|
| ||||||
| Asthma Group (n=8) | ||||||
|
| ||||||
| VO2, L·min−1 | ||||||
| Control trial | 0.40 ± 0.1 | 2.6 ± 0.4 | 2.8 ± 0.6 | 3.1 ± 0.6 | 3.3 ± 0.7 | 0.71 ± 0.2 |
| BD trial | 0.50 ± 0.2 | 2.7 ± 0.4 | 2.8 ± 0.5 | 3.1 ± 0.7 | 3.4 ± 0.6 | 0.81 ± 0.2 |
| SHAM trial | 0.50 ± 0.2 | 2.5 ± 0.6 | 2.7 ± 0.6 | 3.0 ± 0.7 | 3.3 ± 0.8 | 0.69 ± 0.2 |
| BC trial | 0.40 ± 0.1 | 2.5 ± 0.5 | 2.8 ± 0.5 | 3.1 ± 0.7 | 3.2 ± 0.7 | 0.69 ± 0.2 |
| Ve/VO2 | ||||||
| Control trial | 39.4 ± 7.8 | 28.2 ± 3.3 | 30.9 ± 3.5 | 35.0 ± 5.6 | 37.6 ± 7.6 | 48.7 ± 5.0 |
| BD trial | 35.0 ± 10.1 | 27.7 ± 4.5 | 30.5 ± 6.1 | 33.1 ± 7.2 | 36.0 ± 6.6 | 42.5 ± 9.2 |
| SHAM trial | 36.1 ± 12.2 | 28.5 ± 4.0 | 31.9 ± 5.2 | 32.7 ± 4.2 | 38.4 ± 7.1 | 50.0 ± 4.3 |
| BC trial | 37.2 ± 8.0 | 29.2 ± 3.1 | 31.4 ± 3.5 | 33.4 ± 4.9 | 36.8 ± 6.3 | 46.7 ± 6.2 |
| HR, BPM | ||||||
| Control trial | 74.0 ± 13 | 136.5 ± 14 | 151.8 ± 11 | 165.0 ± 13 | 173.3 ± 12 | 110.8 ± 17 |
| BD trial | 81.1 ± 16 | 142.1 ± 15 | 156.9 ± 13 | 167.1 ± 13 | 173.6 ± 13 | 117.3 ± 18 |
| SHAM trial | 72.3 ± 9 | 139.4 ± 16 | 154.9 ± 14 | 165.9 ± 14 | 172.3 ± 13 | 112.8 ± 19 |
| BC trial | 75.3 ± 13 | 139.6 ± 14 | 154.4 ± 13 | 164.7 ± 13 | 168.4 ± 13 | 114.1 ± 28 |
| RPE, Breath | ||||||
| Control trial | 0.25 ± 0.27 | 1.50 ± 1.28 | 3.00 ± 1.85 | 5.13 ± 2.46 | 7.88 ± 1.96 | 3.31 ± 2.69 |
| BD trial | 0.19 ± 0.26 | 2.50 ± 1.41 | 4.13 ± 2.10 | 5.57 ± 2.70 | 7.81 ± 3.21 | 3.19 ± 2.00 |
| SHAM trial | 0.31 ± 0.70 | 2.19 ± 1.13a | 3.56 ± 1.45 | 5.50 ± 1.93 | 7.63 ± 2.13 | 3.00 ± 1.77 |
| BC trial | 0.69 ± 1.36 | 2.13 ± 1.55 | 4.00 ± 2.14 | 5.38 ± 2.67 | 7.31 ± 3.08 | 3.88 ± 3.04 |
| RPE, Legs | ||||||
| Control trial | 0.19 ± 0.26 | 2.19 ± 1.46 | 3.88 ± 1.64 | 6.50 ± 2.00 | 8.63 ± 1.51 | 3.14 ± 2.12 |
| BD trial | 0.13 ± 0.23 | 2.88 ± 1.73 | 4.81 ± 2.10 | 6.31 ± 2.22 | 8.13 ± 1.64 | 2.71 ± 2.48 |
| SHAM trial | 0.13 ± 0.23 | 2.94 ± 1.43 | 4.25 ± 1.58 | 6.25 ± 1.89 | 8.00 ± 2.27 | 2.75 ± 1.16 |
| BC trial | 0.13 ± 0.23 | 2.50 ± 1.77 | 4.56 ± 2.38 | 5.69 ± 2.52 | 8.38 ± 1.69 | 3.50 ± 1.93 |
VO2, oxygen consumption; Ve/VO2, ventilatory equivalent for oxygen consumption; RPE, rating of perceived exertion. a, p<0.05 vs. control trial
DISCUSSION
We sought to determine the effects of variable pre-exercise airway function on the ventilatory responses to whole-body exercise in asthmatic adults. Moreover, all studies were completed by a control group of non-asthmatic subjects. We hypothesized that exercise ventilation would be similar despite variable pre-exercise airway function in asthmatic subjects. Indeed, despite markedly different pre-exercise pulmonary function in asthmatic subjects, exercise ventilation was nearly identical among the four trials. Furthermore, there were no differences in exercise ventilation between control and asthmatic subjects among any of the four trials. These results demonstrate a robust pulmonary system in the mild asthmatic; one that is apparently capable of adequately responding to the acute demand for increased airflow necessitated by high-intensity aerobic exercise. From a clinical standpoint, these findings support the idea that regular exercise may be beneficial for the asthmatic (24, 28).
Exercise workload
The exercise intensity was set at 85% of the peak workrate achieved during an incremental exercise test-to-exhaustion. This demanding exercise workload was both high enough to challenge the capacity of the pulmonary system for generating airflow – reflecting the contractile properties of the respiratory muscles and the airway structural and parenchymal determinants of airflow – but also low enough so that subjects were able to exercise for a duration sufficient to address the purpose of this study. A workload either higher or lower than this would not have met these criteria. Additionally, the exercise workload in this study is very similar to the workload generally employed in studies evaluating exercise-induced bronchoconstriction.
Pre-exercise pulmonary function
In the current study, pulmonary function was used as an indication of the overall state of airway function prior to exercise. To this end, the decreased forced expiratory flow rates prior to exercise during BC and the increased flow rates during BD indicate markedly different states of airway function prior to the exercise in asthmatic subjects (Figure 1B & 1D). Thus, at the onset of exercise during BC, the capacity for generating airflow was severely compromised. Nonetheless, this markedly disparate pre-exercise airflow generating capacity had no effect on the exercise ventilatory response.
In control subjects, pulmonary function increased slightly following bronchodilator and decreased slightly following EVH. In both cases, the changes from BL were statistically significant. Interestingly, pulmonary function also decreased slightly but significantly between BL and PI during both CON and SHAM trials. Thus, the tendency for pulmonary function to decrease with multiple efforts in asthmatics (i.e., spirometry-induced bronchospasm) may also be the case for non-asthmatic subjects (16). We also note that although the magnitude of decrease in pulmonary function during CON and SHAM was less in nonasthmatics, the greater uniformity of response actually resulted in more statistical significance. These findings exemplify the recurring conundrum of distinguishing between statistical vs. physiological significance in human biology. These findings also highlight the fact that both between- and within-subject variability in pulmonary function – and likely other measures of physiologic functioning – will always be greater in a group of asthmatics compared with nonasthmatic counterparts. Given the variability in airway inflammation, AHR, and lung function in the asthmatic, this is not surprising.
Exercise ventilation
To the best of our knowledge, only one previous study has assessed exercise ventilation following both improved (via metaproterenol) and worsened (via methacholine) airway mechanical function in asthmatic subjects (26). Mahler and colleagues reported reduced Vt and V̇e at peak exercise following methacholine challenge compared with a control trial and pre-exercise bronchodilation in adults with reversible obstructive airway disease. In the current study, exercise ventilation was not affected by variable pre-exercise airway mechanical function. There are several differences between the current study and the study by Mahler and colleagues. Most notably, subjects in the study by Mahler were both older and had more severe airways obstruction than our subjects. Secondly, the methods for inducing bronchoconstriction are very different between the two studies; in one case a direct-acting bronchiolar smooth muscle agonist was used (i.e., cholinergic agonist, methacholine) whereas in the current study airway narrowing was induced by the indirect inflammatory effects of dry gas hyperpnea.
Although airway resistance is determined by interactions among several variables, it is most sensitive to changes in airway radius (9). Considering this importance of airway diameter in determining airflow, it is reasonable to posit a relationship between baseline airway function and exercise ventilation. Evidence that supports this hypothesis, however, is limited. Previous investigations have assessed the effects of pre-exercise bronchodilation on exercise ventilation and exercise capacity in asthmatic adults (11, 18, 35, 36, 37). In all of these studies, pre-exercise bronchodilation had no effect on exercise ventilation as compared with control and placebo conditions. In fact, the airways of asthmatic subjects undergo an initial bronchodilation during whole-body exercise (4, 10, 15, 38). Thus, the natural bronchodilation with exercise may obviate the potential for altered airway function – whether improved or impaired – to influence exercise ventilation. An interesting experiment would be to prevent bronchodilation during exercise in asthmatic subjects. Presumably, this would be difficult, however, as exercise bronchodilation still occurs when the bronchoconstrictors histamine and methacholine are inhaled during exercise in the asthmatic (19, 38). A gradual bronchoconstriction can occur over time during moderate intensity exercise lasting longer than ~10 minutes (4, 21), but the effects of this gradual bronchoconstriction on exercise ventilation and performance have not been studied.
In this study, subjects exercised after six minutes of voluntary eucapnic hyperpnea. Voluntary hyperpnea stimulates the release of inflammatory mediators from resident (i.e., epithelial) and non-resident (i.e., mast cells) airway cells (1, 22). In the asthmatic, this non-specific stimulus causes bronchiolar smooth muscle contraction, plasma exudation, airway wall edema, and mucus secretion into the airways. The stimulus for this inflammatory-based airway narrowing with voluntary hyperpnea is thought to be identical to the osmotically-based stimulus for airway narrowing following whole-body exercise. In this study, subjects began exercise within 15 minutes following the EVH challenge, well before airway narrowing and inflammation had resolved.
A major finding of this study is that exercise ventilation was not affected by the poor airway function prior to exercise. In previous studies, asthmatic subjects have exercised following a variety of procedures that cause airway narrowing, including inhaled histamine, aerosolized hypertonic saline, methacholine, and EVH (5, 6, 13, 25). The purpose of these investigations, however, was to study bronchoconstriction following exercise, and analyses of exercise ventilation were minimal. Moreover, exercise was not commenced until pulmonary function had returned, or nearly returned, to baseline values; exercise began between 30 minutes and six hours following the pre-exercise challenge. In the current study, exercise was commenced within 10-15 minutes of the EVH challenge, when FEV1.0 was still significantly reduced by the challenge.
In one previous study, asthmatic subjects completed an exercise challenge during the midst of a spontaneous asthma attack and also during a late asthmatic reaction to inhaled allergen (10). Exercise ventilation and V̇O2peak were preserved during both exercise conditions. In a different study, exercise ventilation was compared between two exercise bouts in which pulmonary function preceding the second bout was decreased compared with the first (15). Exercise ventilation during the second bout was identical to the first bout. The current findings support and extend these observations.
Exercise lung volumes
There were no statistically significant differences in operating lung volumes in either control or asthmatic subjects. In asthmatic subjects, the average 0.6 L difference in pre-exercise IC between BC and the other three trials, though not statistically significant, is certainly of some physiological significance. This finding is in agreement with other studies demonstrating a decreased IC with bronchoconstriction in asthmatic subjects (31, 39). An increased end-expiratory lung volume (i.e., dynamic hyperinflation) with bronchoconstriction likely represents a compensatory response to increase airway diameter or reduce airway closure, due to interdependence between the airways and lung parenchyma (7). Importantly, the decreased pre-exercise IC during BC was not maintained during exercise, as exercise IC increased progressively to equal the values seen during the other three trials by end exercise. The slight differences in resting Vt/IC between BC and the other trials also disappeared during exercise. Collectively, these results demonstrate a robustly “agile” pulmonary system in the asthmatic, with a wonderful ability to respond to the requirement for increased airflow during exercise.
Perceptual responses during exercise
Despite the differences in pre-exercise airway function among trials in asthmatic subjects, both breathing and leg discomfort were the same during all four exercise trials. Perhaps most interesting is the unchanged RPE-breath following EVH. Intuitively, breathing discomfort would be expected to increase following bronchoconstriction. In asthmatic subjects at rest, however, the relationships between airway function and perception of breathlessness are unclear (30). In one study, pre-exercise bronchoconstriction via methacholine did cause an increased intercept for the relationship between exercise workrate and RPE-breath (26). In the same study, however, there were no changes in slope or intercept between RPE-breath and all other exercise variables, including flowrate, mouth pressure, breathing frequency, or tidal volume. Perceptual responses to exercise result from a complicated amalgamation of multiple ascending and descending neurohumerol inputs (27). Further investigation is needed to improve our understanding of the determinants of effort perception during exercise, and how perception of these determinants may be altered in disorders of the pulmonary system.
Generalizability of findings
The findings in this study are limited to asthmatic adults with primarily mild disease. Subjects were able to increase ventilation during exercise no matter how compromised airway function was prior to exercise. In asthmatics with more severely inflamed airways or greater degrees of airflow limitation, the ability to increase ventilation during exercise will likely be more limited.
In the current study, exercise consisted of three minutes at 70% peak power output followed by exercise-to-exhaustion at 85% peak power output. The current findings are thus confined to the high intensity, relatively short duration exercise completed by subjects in this study. There are a variety of bronchodilatory and bronchconstricting factors that interact to determine airway caliber during exercise in the asthmatic (3), and the balance of these factors will vary depending on exercise duration and intensity. For example, bronchoconstriction can occur during submaximal exercise lasting ten minutes or longer and also during variable intensity exercise when exercise workload is decreased (4, 21). The current findings of unchanged exercise ventilation despite altered pre-exercise airway function should not be generalized to all intensities and durations of aerobic exercise. Further studies will be necessary to determine the relationships among airway function, exercise ventilation, and exercise workload and duration in asthmatic subjects.
Conclusions
We evaluated the effects of both improved and worsened airway function on exercise ventilation in asthmatic adults and compared the results with nonasthmatic control subjects. In a group of mild asthmatics, exercise ventilation was not affected by significant alterations in pre-exercise airway function. Notably, exercise ventilation was nearly indistinguishable between the asthmatic and control subjects. These findings corroborate previous studies demonstrating unchanged exercise ventilation and capacity following pre-exercise bronchodilation in the asthmatic. Moreover, these results demonstrate a remarkable dissociation between pre-exercise airway function and the exercise ventilatory response in the mildly asthmatic adult. These findings also contrast with the general notion that baseline airway function influences exercise ventilation in the asthmatic.
ACKNOWLEDGMENTS
Support made possible by the Vermont Genetics Network through Grant Number 8P20GM103449 from the INBRE Program of the National Institute of General Medical Sciences (NIGMS) and the National Center for Research Resources (NCRR), components of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH. The results of this manuscript do not constitute endorsement by the American College of Sports Medicine.
Footnotes
CONFLICTS OF INTEREST Matthew Rossman does not have any conflicts of interest to report
Susan Nader does not have any conflicts of interest to report
Dustin Berry does not have any conflicts of interest to report
Francesca Orsini does not have any conflicts of interest to report
Andrew Klansky does not have any conflicts of interest to report
Hans Haverkamp does not have any conflicts of interest to report
REFERENCES
- 1.Anderson SD. Indirect challenge tests: Airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest. 2010;138:25S–30S. doi: 10.1378/chest.10-0116. [DOI] [PubMed] [Google Scholar]
- 2.Anderson SD, Brannan JD. Methods for “indirect” challenge tests including exercise, eucapnic voluntary hyperpnea, and hypertonic aerosols. Clin Rev Allergy Immunol. 2003;24:27–54. doi: 10.1385/CRIAI:24:1:27. [DOI] [PubMed] [Google Scholar]
- 3.Beck KC. Control of airway function during and after exercise in asthmatics. Med Sci Sports Exerc. 1999;31:S4–11. doi: 10.1097/00005768-199901001-00002. [DOI] [PubMed] [Google Scholar]
- 4.Beck KC, Offord KP, Scanlon PD. Bronchoconstriction occurring during exercise in asthmatic subjects. Am J Respir Crit Care Med. 1994;149:352–357. doi: 10.1164/ajrccm.149.2.8306029. [DOI] [PubMed] [Google Scholar]
- 5.Belcher NG, Rees PJ, Clark TJ, Lee TH. A comparison of the refractory periods induced by hypertonic airway challenge and exercise in bronchial asthma. Am Rev Respir Dis. 1987;135:822–825. doi: 10.1164/arrd.1987.135.4.822. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Dov I, Gur I, Bar-Yishay E, Godfrey S. Refractory period following induced asthma: contributions of exercise and isocapnic hyperventilation. Thorax. 1983;38:849–853. doi: 10.1136/thx.38.11.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown RH, Scichilone N, Mudge B, Diemer FB, Permutt S, Togias A. High-resolution computed tomographic evaluation of airway distensibility and the effects of lung inflation on airway caliber in healthy subjects and individuals with asthma. Am J Respir Crit Care Med. 2001;163:994–1001. doi: 10.1164/ajrccm.163.4.2007119. [DOI] [PubMed] [Google Scholar]
- 8.Choi IS, Ki WJ, Kim TO, Han ER, Seo IK. Seasonal factors influencing exercise-induced asthma. Allergy Asthma Immunol Res. 2012;4:192–198. doi: 10.4168/aair.2012.4.4.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comroe J. Physiology of Respiration. 2nd ed Year Book Medical Publishers; Chicago (IL): 1974. pp. 117–128. [Google Scholar]
- 10.Crimi E, Pellegrino R, Smeraldi A, Brusasco V. Exercise-induced bronchodilation in natural and induced asthma: effects on ventilatory response and performance. J Appl Physiol. 2002;92:2353–2360. doi: 10.1152/japplphysiol.01248.2001. [DOI] [PubMed] [Google Scholar]
- 11.Freeman W, Packe GE, Cayton RM. Effect of nebulised salbutamol on maximal exercise performance in men with mild asthma. Thorax. 1989;44:942–947. doi: 10.1136/thx.44.11.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2012:22–27. Available from: http://www.ginasthma.org/
- 13.Hamielec CM, Manning PJ, O’Byrne PM. Exercise refractoriness after histamine inhalation in asthmatic subjects. Am Rev Respir Dis. 1988;138:794–798. doi: 10.1164/ajrccm/138.4.794. [DOI] [PubMed] [Google Scholar]
- 14.Hargreave FE, Nair P. The definition and diagnosis of asthma. Clin Exp Allergy. 2009;39:1652–1658. doi: 10.1111/j.1365-2222.2009.03321.x. [DOI] [PubMed] [Google Scholar]
- 15.Haverkamp HC, Dempsey JA, Miller JD, et al. Repeat exercise normalizes the gasexchange impairment induced by a previous exercise bout in asthmatic subjects. J Appl Physiol. 2005;99:1843–1852. doi: 10.1152/japplphysiol.01399.2004. [DOI] [PubMed] [Google Scholar]
- 16.Holcroft CA, Eisen EA, Sama SR, Wegman DH. Measurement characteristics of peak expiratory flow. Chest. 2003;124:501–510. doi: 10.1378/chest.124.2.501. [DOI] [PubMed] [Google Scholar]
- 17.Holgate ST, Arshad HS, Roberts GC, Howarth PH, Thurner P, Davies DE. A new look at the pathogenesis of asthma. Clin Sci (Lond) 2010;118:439–450. doi: 10.1042/CS20090474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ienna TM, McKenzie DC. The asthmatic athlete: metabolic and ventilatory responses to exercise with and without pre-exercise medication. Int J Sports Med. 1997;18:142–148. doi: 10.1055/s-2007-972610. [DOI] [PubMed] [Google Scholar]
- 19.Inman MD, Watson RM, Killian KJ, O’Byrne PM. Methacholine airway responsiveness decreases during exercise in asthmatic subjects. Am Rev Respir Dis. 1990;141:1414–1417. doi: 10.1164/ajrccm/141.6.1414. [DOI] [PubMed] [Google Scholar]
- 20.Jindal SK, Aggarwal AN, Gupta D. Diurnal variability of peak expiratory flow. J Asthma. 2002;39:363–373. doi: 10.1081/jas-120004029. [DOI] [PubMed] [Google Scholar]
- 21.Johnson BD, Scanlon PD, Beck KC. Regulation of ventilatory capacity during exercise in asthmatics. J Appl Physiol. 1995;79:892–901. doi: 10.1152/jappl.1995.79.3.892. [DOI] [PubMed] [Google Scholar]
- 22.Kippelen P, Larsson J, Anderson SD, Brannan JD, Dahlen B, Dahlen SE. Effect of sodium cromoglycate on mast cell mediators during hyperpnea in athletes. Med Sci Sports Exerc. 2010;42:1853–1860. doi: 10.1249/MSS.0b013e3181da4f7d. [DOI] [PubMed] [Google Scholar]
- 23.Lareau SC, ZuWallack R, Carlin B, et al. Pulmonary Rehabilitation. Am J Resp Crit Care Med. 1999;159:1666–1682. [Google Scholar]
- 24.Lucas SR, Platts-Mills TA. Physical activity and exercise in asthma: relevance to etiology and treatment. J Allergy Clin Immunol. 2005;115:928–934. doi: 10.1016/j.jaci.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 25.Magnussen H, Reuss G, Jorres R. Airway response to methacholine during exercise induced refractoriness in asthma. Thorax. 1986;41:667–670. doi: 10.1136/thx.41.9.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahler DA, Faryniarz K, Lentine T, Ward J, Olmstead EM, O’Connor GT. Measurement of breathlessness during exercise in asthmatics. Predictor variables, reliability, and responsiveness. Am Rev Respir Dis. 1991;144:39–44. doi: 10.1164/ajrccm/144.1.39. [DOI] [PubMed] [Google Scholar]
- 27.Mahler DA, Gustavo R, Baird JC. Mechanisms and measurement of exertional dyspnea. In: Weisman IM, Zeballos RJ, editors. Clinical Exercise Testing. Karger; New York: 2002. pp. 72–80. [Google Scholar]
- 28.Mendes FA, Almeida FM, Cukier A, et al. Effects of aerobic training on airway inflammation in asthmatic patients. Med Sci Sports Exerc. 2011;43:197–203. doi: 10.1249/MSS.0b013e3181ed0ea3. [DOI] [PubMed] [Google Scholar]
- 29.Miller MR, Hankinson J, Brusasco V, et al. Standardization of Spirometry. Eur Resp J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 30.Ottanelli R, Rosi E, Romagnoli I, et al. Perception of bronchoconstriction and bronchial hyper-responsiveness in asthma. Clin Sci (Lond) 2000;98:681–687. [PubMed] [Google Scholar]
- 31.Pellegrino R, Violante B, Nava S, Rampulla C, Brusasco V, Rodarte JR. Expiratory airflow limitation and hyperinflation during methacholine-induced bronchoconstriction. J Appl Physiol. 1993;75:1720–1727. doi: 10.1152/jappl.1993.75.4.1720. [DOI] [PubMed] [Google Scholar]
- 32.Pescatello LS, Arena R, Riebe D, Thompson PD. ACSM’s guidelines for exercise testing and prescription. 9th ed Wolters Kluwer and Lippincott Williams & Wilkins; Baltimore (MD): 2014. pp. 331–334. [Google Scholar]
- 33.Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16:5–40. [PubMed] [Google Scholar]
- 34.Ram FS, Robinson SM, Black PN, Picot J. Physical training for asthma. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD001116.pub2. CD001116. [DOI] [PubMed] [Google Scholar]
- 35.Revill SM, Morgan MD. The cardiorespiratory response to submaximal exercise in subjects with asthma following pretreatment with controlled release oral salbutamol and highdose inhaled salmeterol. Respir Med. 1998;92:1053–1058. doi: 10.1016/s0954-6111(98)90354-7. [DOI] [PubMed] [Google Scholar]
- 36.Robertson W, Simkins J, O’Hickey SP, Freeman S, Cayton RM. Does single dose salmeterol affect exercise capacity in asthmatic men? Eur Respir J. 1994;7:1978–1984. [PubMed] [Google Scholar]
- 37.Steinshamn S, Sandsund M, Sue-Chu M, Bjermer L. Effects of montelukast and salmeterol on physical performance and exercise economy in adult asthmatics with exercise-induced bronchoconstriction. Chest. 2004;126:1154–1160. doi: 10.1378/chest.126.4.1154. [DOI] [PubMed] [Google Scholar]
- 38.Stirling DR, Cotton DJ, Graham BL, Hodgson WC, Cockcroft DW, Dosman JA. Characteristics of airway tone during exercise in patients with asthma. J Appl Physiol. 1983;54:934–942. doi: 10.1152/jappl.1983.54.4.934. [DOI] [PubMed] [Google Scholar]
- 39.Tantucci C, Ellaffi M, Duguet A, et al. Dynamic hyperinflation and flow limitation during methacholine-induced bronchoconstriction in asthma. Eur Respir J. 1999;14:295–301. doi: 10.1183/09031936.99.142. [DOI] [PubMed] [Google Scholar]
- 40.Welsh L, Kemp JG, Roberts RG. Effects of physical conditioning on children and adolescents with asthma. Sports Med. 2005;35:127–141. doi: 10.2165/00007256-200535020-00003. [DOI] [PubMed] [Google Scholar]