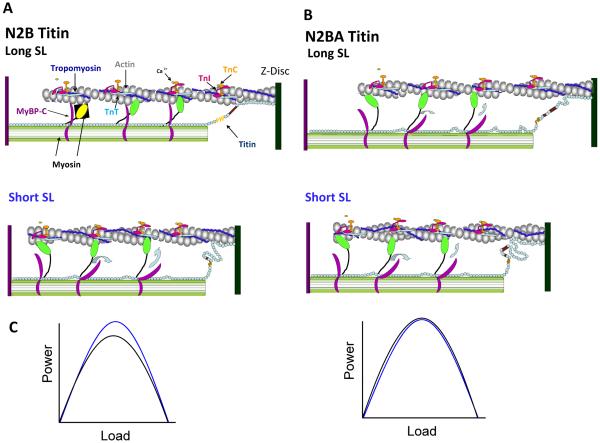
Figure 1.
Myofibrillar model for altered cross-bridge kinetics mediated by sarcomere length and titin. A. Representation of a half-sarcomere containing N2B titin at two different sarcomere lengths. At long sarcomere length titin is extended and provides passive tension that may help maintain extension of MyBP-C from the thick filament to constrain the myosin cross-bridges. At short sarcomere length, titin is slackened and this coincident decrease in passive tension may alleviate MyBP-C constraint of the cross-bridge, leading to more flexible crossbridges that act to more readily retain thin filament activation during active shortening. B. Half sarcomere depiction in the presence of N2BA titin. Even at long sarcomere length titin is slightly slackened, which would increase flexibility of the cross-bridges and sustain thin filament activation allowing more cross-bridges to work against the load yielding less load per bridge so each cross-bridge cycles faster. C. The augmented shortening speed at long sarcomere length in N2BA myofibrils is predicted to eliminate faster loaded shortening at short sarcomere length (right panel) observed in N2B-containing myofibrils (left panel) when force is matched between long and short SL. (Short SL is represented by the blue line; long SL is represented by the black line.) (Sarcomeric protein and protein domains are not drawn to scale.)