Abstract
Purpose
The expression of glucose transporter-1 (Glut-1) gene and those of major thyroid-specific genes were examined in papillary carcinoma tissues, and the expressions of these genes were compared with cancer differentiation grades.
Materials and Methods
Twenty-four human papillary carcinoma tissues were included in this study. The expressions of Glut-1- and thyroid-specific genes [sodium/iodide symporter (NIS), thyroid peroxidase, thyroglobulin, TSH receptor and pendrin] were analyzed by RT-PCR. Expression levels were expressed as ratios versus the expression of beta-actin. Pathologic differentiation of papillary carcinoma was classified into a relatively well-differentiated group (n = 13) and relatively less differentiated group (n = 11).
Results
Glut-1 gene expression was significantly higher in the less differentiated group (0.66 ± 0.04) than in the well-differentiated group (0.59 ± 0.07). The expression levels of the NIS, PD and TG genes were significantly higher in the well-differentiated group (NIS: 0.67 ± 0.20, PD: 0.65 ± 0.21, TG: 0.74 ± 0.16) than in the less differentiated group (NIS: 0.36 ± 0.05, PD: 0.49 ± 0.08, TG: 0.60 ± 0.11), respectively. A significant negative correlation was found between Glut-1 and NIS expression, and positive correlations were found between NIS and TG, and between NIS and PD.
Conclusion
The NIS, PD and TG genes were highly expressed in well-differentiated thyroid carcinomas, whereas the Glut-1 gene was highly expressed in less differentiated thyroid carcinomas. These findings provide a molecular rationale for the management of papillary carcinoma, especially in the selection of FDG PET or radioiodine whole-body scan and I-131-based therapy.
Keywords: Glucose transporter-1, Sodium/iodide symporter, Thyroid peroxidase, Thyroglobulin, TSH receptor, Pendrin, Papillary thyroid cancer
Introduction
Iodide enters the thyroid follicular cells via a specific sodium-dependent transporter, the sodium/iodide symporter (NIS), which is located on the basolateral cell membrane. Cellular iodine accumulation is also regulated by thyrotropin receptor (TSH-R), which represents a crucial and rate-limiting step in the biosynthesis of thyroid hormone. Moreover, iodine is incorporated into thyroid hormones through a series of metabolic steps that include the proteins pendrin (PD), thyroid peroxidase (TPO) and thyroglobulin (TG) [1], and these proteins are regarded as differentiation markers of thyroid follicular cells. A defective iodide-trapping mechanism due to reduced NIS expression appears to be an early and consistent feature of the oncogenic transformation of thyroid cells, and other changes in the expressions of thyroid-specific markers (TG, TPO, TSH-R, PD) have also been variably associated with neoplastic transformation [2].
Cancer cells demonstrate increased glucose uptake and utilization, which may be caused by the overexpression of glucose transporters and increased activity of glycolytic enzymes. Of these, glucose transporter-1 (Glut-1) appears to be mainly involved in the oncogenesis of many malignant tumors including thyroid cancer [3, 4]. Glut-1 overexpression has been founded to be expressed in malignant thyroid tumors [4–6] with respect to benign tumors, but a role in carcinogenesis has not been postulated.
Several nuclear medicine imaging modalities have been used to detect recurrent and metastatic differentiated thyroid cancers postoperatively. Radioiodine whole-body scans have been widely used for more than 60 years, but recently, F-18 fluorodeoxyglucose (FDG) PET has emerged as a new valuable imaging method for thyroid cancer. In particular, FDG PET imaging is considered useful for the detection of recurrent or metastatic cancer in patients with negative radioiodine scans [7, 8]. The expressions of NIS and Glut-1 are related to the accumulation of radioiodine and F-18 FDG in thyroid cancer cells [9–11], and the expressions of these genes may provide a molecular biologic basis for these image modalities. However, to the best of our knowledge, only one report [12] has been issued on the relationships between the expressions of Glut-1 and NIS or other thyroid-specific genes in differentiated thyroid cancer. Thus, we investigated the expressions of Glut-1 and major thyroid-specific genes in human papillary carcinoma tissues, and compared their expressions with cancer differentiation grades.
Materials and Methods
Thyroid Samples
All tissues were obtained during thyroid surgery at Seoul National University Hospital. The protocol for the current study was reviewed and approved by the institutional review board of Seoul National University Hospital. Tissues were immediately frozen and stored at −70 °C until required. Papillary carcinomas were defined using the pathologic criteria described by the World Health Organization (WHO) [13]. The tissues of 24 papillary carcinoma cases were included in this study. The expressions of Glut-1 and five thyroid-specific genes (NIS, TPO, TG, TSH-R and PD) were analyzed by RT-PCR.
Histopathologic Evaluation
Histologic slides were reviewed by two pathologists unaware of the clinical data. Histologic patterns of the papillary carcinomas were classified into two groups: (1) a relatively well-differentiated group and (2) relatively less differentiated group. The relatively well-differentiated group had cytologic features as follows: well-formed dendritical papillae or broad papillae with a fibrovascular core, and typical features including a nuclear groove, pseudo-inclusion and empty appearance [13]. The relatively well-differentiated group occasionally contained follicular arrangements or a focal solid pattern (Fig. 1a). On the other hand, although the relatively less differentiated group also had the typical nuclei of papillary carcinoma, group members were characterized by trabecular, diffusely follicular or diffusely sclerosing features in comparison with the relatively well-differentiated group. Tumor cells of the relatively less differentiated group frequently showed oxyphilic change (oxyphilic or columnar cells with abundant eosinophilic cytoplasm), accompanied with/without nuclear atypia (Fig. 1b).
Fig. 1.
Representative photomicrographs of relatively well-differentiated (a) and relatively less differentiated (b) papillary thyroid cancer (H&E, ×200). In relatively well-differentiated cancer, well-developed papillary structures with a fibrovascular core, typical nuclear features and scant cytoplasm were observed. In relatively less differentiated cancer, trabecular or tubular growth patterns with prominent tumor cell oxyphilic changes were observed
RNA Extraction and RT-PCR
Total cellular RNA was obtained from microdissected snap-frozen tissues by phenol–guanidine–isothiocyanate extraction using Trizol kits (Gibco BRL, Gaithersburg, MD). The purity of total RNA was assessed using the ratio of optical densities at 260 and 280 nm and by the absence of contaminating DNA bands by agarose electrophoresis. cDNA synthesis was performed using 1 μg of total RNA, 20 pmol of oligo dT primer and 200 U of recombinant Maloney-murine leukemia virus reverse transcriptase (Clontech Inc., Palo Alto, CA), as instructed by the manufacturer. Oligonucleotide primers, including those of β-actin cDNA [14], and the conditions used for amplifying NIS cDNA, TG cDNA [15], TPO cDNA [16], TSH-R cDNA [17], PD [18] and Glut-1 cDNA are shown in Tables 1 and 2. RT-PCR products were observed in 1.4 % agarose gel stained with ethidium bromide. The bands on positive film were scanned by densitometry, and the densities and widths were determined by Tina2.1 software (Raytest, Straubengardt, Germany) to calculate product expressions versus β-actin. Negative PCR controls contained the respective primers without cDNA.
Table 1.
Primers in PCR
| Sequence (sense) | Position of primer in cDNA | sequence (antisense) | Position of primer in cDNA | Product (bp) | |
|---|---|---|---|---|---|
| NIS | TCTCTCAGTCAACGCCTCT | 1802-1820 | ATCCAGGATGGCCACTTCTT | 2080-2099 | 297 |
| TG | GGCTAATGCTACATGTCCTG | 5316-5335 | GCTTCTGTTGGAGATGCTGG | 5527-5546 | 230 |
| TPO | GTCTGTCACGCTGGTTATGG | 110-129 | CAATCACTCCGCTTGTTGGC | 332-351 | 241 |
| TSH-R | TGAAGCTGTACAACAACGGC | 589-608 | TCAGTTCCTTCAGGTGCTCC | 782-801 | 212 |
| Pendrin | CACAGTTGGATTTGATGCCATTAGAGTA | 1928-1955 | TACGCATAGCCTCATCCTGGACATC | 2529-2553 | 625 |
| Glut-1 | TACCCTGGATGTCCTATCTG | 1261-1280 | CACACAGTTGCTCCACATAC | 1448-1467 | 206 |
| N-ras | CAAGTGGTTATAGATGGTGA | 127-146 | AGGAAGCCTTCGCCTGTCCT | 217-236 | 109 |
| β-actin | TGACGGGGTCACCCACACTGTGCCCATCTA | 541-570 | GAAGCATTTGCGGTGGACGATGGAGGG | 1172-1198 | 657 |
Table 2.
PCR amplification conditions
| hNIS | TPO/TG | TSH-R | PD | Glut-1 | |
|---|---|---|---|---|---|
| Early denaturation | 95 °C (10 min) | 94 °C (10 min) | 95 °C (10 min) | 95 °C (10 min) | 95 °C (10 min) |
| Denaturation | 95 °C (30 s) | 95 °C (30 s) | 95 °C (30 s) | 95 °C (30 s) | 95 °C (30 s) |
| Annealing | 47 °C (30 s) | 57 °C (30 s) | 54 °C (30 s) | 50 °C (30 s) | 54 °C (30 s) |
| Extension | 72 °C (30 s) | 72 °C (30 s) | 72 °C (30 s) | 72 °C (30 s) | 72 °C (1 min) |
| Final extension | 72 °C (10 min) | 72 °C (10 min) | 72 °C (10 min) | 72 °C (10 min) | 72 °C (7 min) |
| Cycles | 30 cycles | 35 cycles | 35 cycles | 30 cycles | 30 cycles |
Statistical Methods
Statistical analysis of mRNA expression levels was performed using the unpaired Student’s t-test. Relationships between expression profiles were investigated using Spearman’s rank order correlation coefficients. P values of <0.05 were considered statistically significant.
Results
Grade of Differentiation
Of the 24 papillary carcinoma tissue samples, 13 were allocated to the relatively well-differentiated group and 11 to the relatively less differentiated group.
Patterns of mRNA Expression
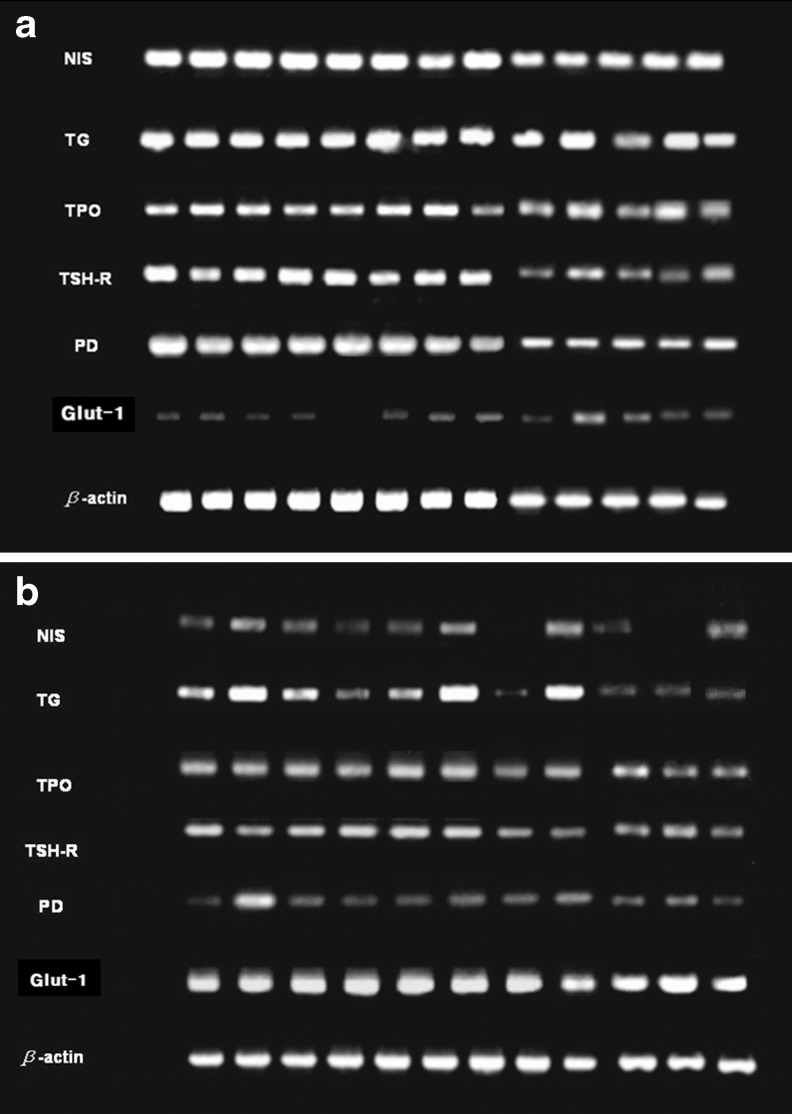
Totally, no Glut-1 expression was observed in one case and no NIS mRNA expression in two cases. The other thyroid-specific genes (TG, TPO, TSH–R and PD) were expressed in all cases (Fig. 2a, 2B).
Fig. 2.
RT-PCR image of each gene in relatively well-differentiated (a) and relatively less differentiated (b) papillary carcinoma
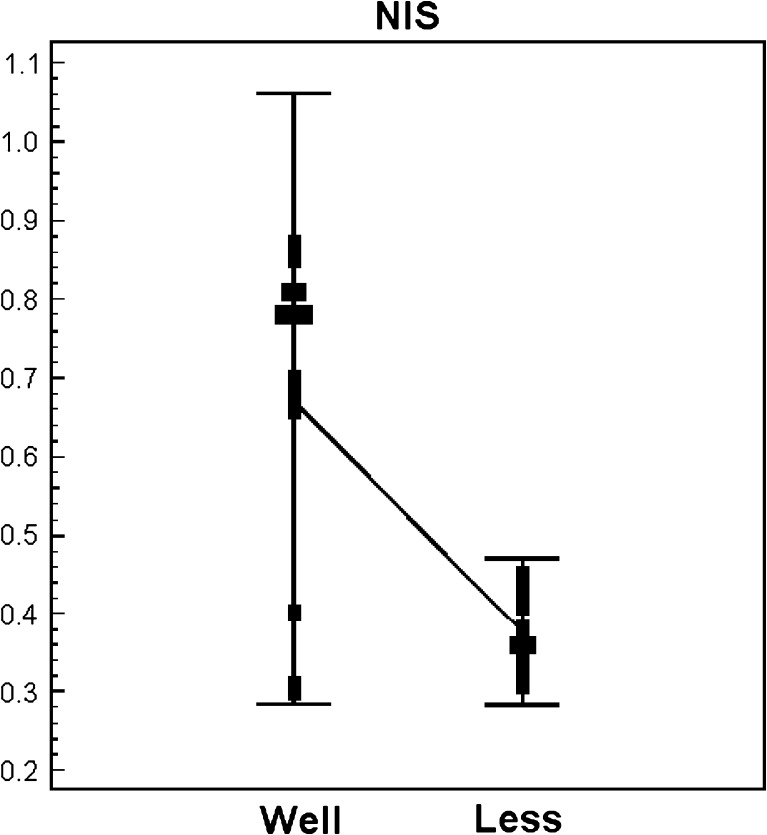
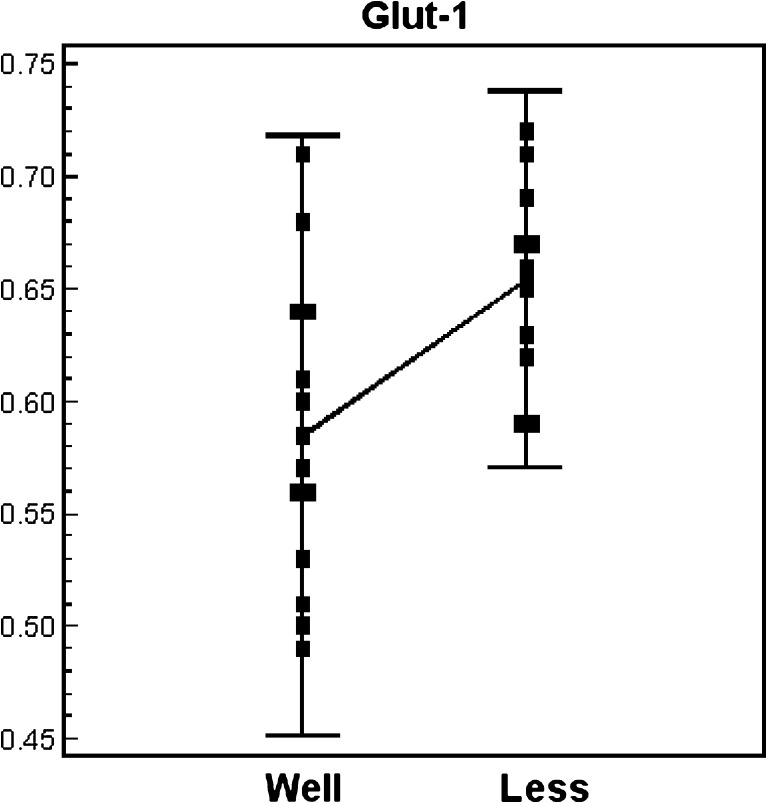
The expressions of the PCR products of NIS, TG, TPO, TSH-R, PD and Glut-1 mRNAs in the relatively well-differentiated and relatively less differentiated groups are shown in Table 3. A wide range of variability was observed between two groups. The expression level of NIS, TG and PD were higher in the relatively well-differentiated group than in the relatively less differentiated group. On the contrary, the relatively well-differentiated group presented lower level of Glut-1 expression (Table 3). A remarkable difference was observed for NIS and Glut-1 mRNA expressions, NIS was more expressed in the relatively well-differentiated group (Fig. 3), and Glut-1 mRNA was more expressed in the relatively less differentiated group, respectively (Fig. 4).
Table 3.
The expression ratios of Glut-1 and thyroid-specific genes versus beta-actin gene in relatively well-differentiated and relatively less differentiated groups
| Well-differentiated group (n = 13) | Less differentiated group (n = 11) | P value (paired t-test) | |
|---|---|---|---|
| NIS | 0.67 (0.20) | 0.36(0.05) | *0.0001 |
| 0.78 [0.30-0.87] | 0.36[0.31-0.45] | ||
| TG | 0.74 (0.16) | 0.60 (0.11) | *0.039 |
| 0.71 [0.56-0.99] | 0.65 [0.42-0.75] | ||
| TPO | 0.56 (0.18) | 0.49 (0.18) | 0.44 |
| 0.58 [0.22-0.89] | 0.47 [0.28-0.87] | ||
| TSH-R | 0.48 (0.27) | 0.39 (0.20) | 0.13 |
| 0.32 [0.16-0.84] | 0.23 [0.13-0.73] | ||
| PD | 0.65 (0.21) | 0.49 (0.08) | *0.036 |
| 0.69 [0.34-0.94] | 0.44 [0.43-0.68] | ||
| Glut-1 | 0.59 (0.07) | 0.66 (0.04) | *0.0027 |
| 0.57 [0.49-0.71] | 0.67 [0.59-0.72] | ||
Mean (SD); median [minimum-maximum]. Results are expressed as a ratio of the beta-actin, n: number of samples
*Statistically significant
Fig. 3.
Expression level of the NIS gene in relatively well-differentiated and relatively less differentiated papillary carcinoma
Fig. 4.
Expression level of the Glut-1 gene in relatively well-differentiated and relatively less differentiated papillary carcinoma
Correlation of Gene Expression Levels
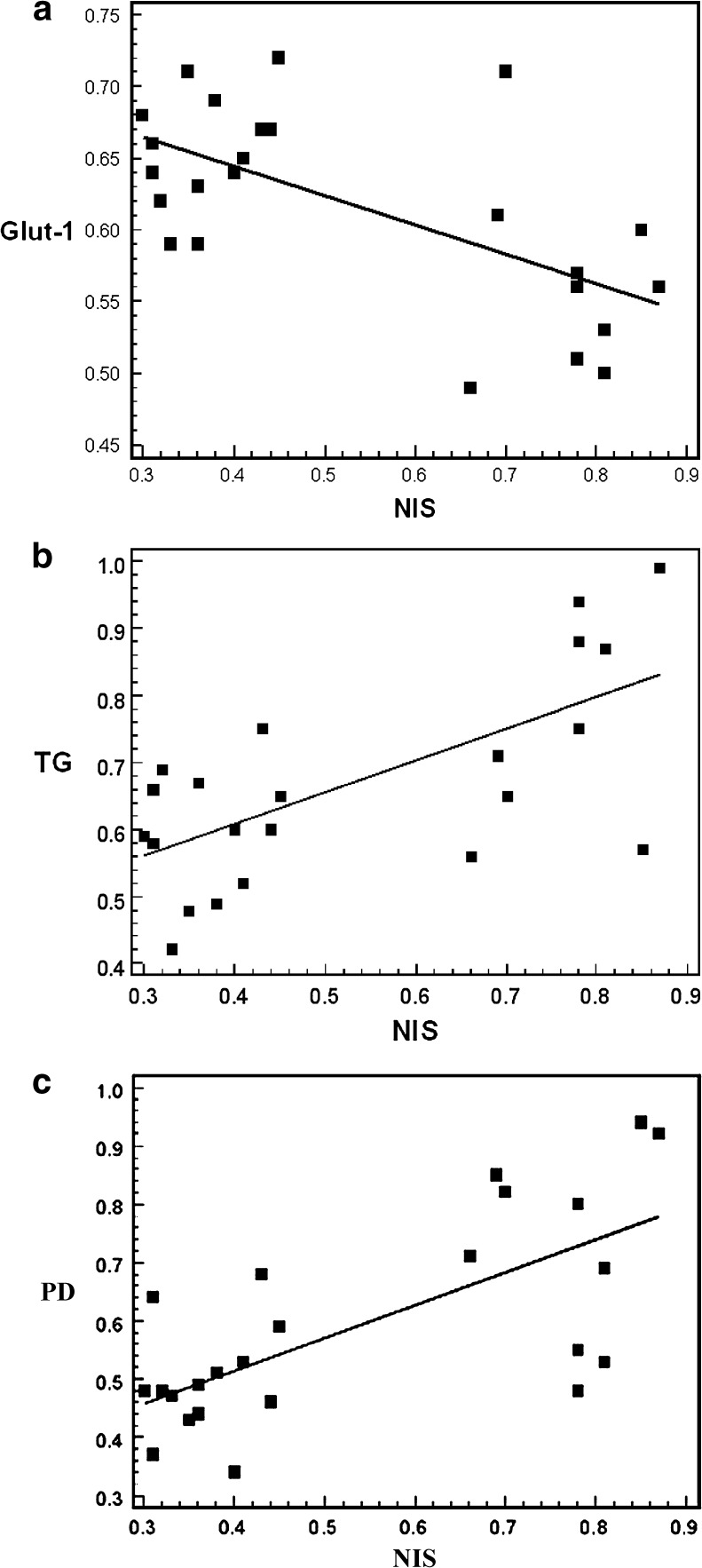
A significant negative correlation was observed between NIS and Glut-1 gene expression (r = −0.60, p < 0.01) (Fig. 5a). Of the thyroid-specific genes, significant positive correlations were found between NIS and TG (r = 0.52, p < 0.05) (Fig. 5b) and NIS and PD (r = 0.77, p < 0.05) (Fig 5c).
Fig. 5.
Correlations of the gene expressions of NIS and Glut-1 (a), NIS and TG (b), and NIS and PD (c)
Discussion
Radioiodine whole-body scans and I-131 therapy have been widely used to manage patients with differentiated thyroid cancer. However, the ability of thyroid cancer cells to concentrate iodide is dependent upon the functional integrity of NIS [8]. Although most differentiated thyroid cancers retain this iodide-concentrating ability, some cancers are incapable of concentrating iodide, which renders them nonsensitive to I-131 therapy and reduces response to therapy [19]. Cartro et al. [20] determined NIS expression at the protein levels by immunohistochemical staining in primary thyroid tumors and found a relation between radioiodine uptake in known metastatic disease and NIS expression in individual patients. In a previous study, we evaluated NIS protein expression by immunostaining in primary thyroid tumors and found that patients positive for NIS expression responded to I-131 therapy better than those negative for NIS [21].
In the present study, the NIS gene was found to be expressed in all but two cases, and its gene expression was higher in the relatively well-differentiated group than in the relatively less differentiated group. Several investigators have reported low NIS gene and protein expression in differentiated thyroid cancers [12, 20, 22–24], and others that papillary cancer tissues display markedly lower and restricted ranges of NIS mRNA expression than normal tissues [12, 20]. Ward et al. [23] identified that patients with papillary thyroid cancer who experienced early recurrence and/or metastasis showed low levels of NIS mRNA expression in the primary tumor. This finding may support our observation that gene expression was higher in the relatively well-differentiated group than in the relatively less differentiated group.
The synthesis of thyroid hormones in TG molecules requires a complex set of reactions. The iodide ion is first transported across the thyrocyte membrane by NIS and then crosses the apical membrane by a PD to reach the follicular colloid, where iodide organification and coupling take place to form the thyroid hormones. These reactions are catalyzed by TPO under oxidative conditions [1]. In differentiated thyroid cancer cells, multiple defects occur in this machinery, which prevent successful thyroid hormone synthesis [2].
In the present study, we found that the TG, TPO, TSH-R and PD genes were expressed in all samples and that their expression levels declined as dedifferentiation progressed. Gerard et al. [24] reported high TG and TSH-R protein expression in thyroid cancers, and low or absent PD and TPO protein expression in papillary carcinomas. Arturi et al. [25] demonstrated that PD expression is reduced or absent in differentiated thyroid cancer tissues, whereas Tanaka et al. [26] reported the presence of TPO gene expression in all thyroid cancer tissues. These minor discrepancies may be due to differences in gene expression and protein synthesis or to a difference in RT-PCR primer cDNA. Lazar et al. [12] observed that NIS gene expression in thyroid carcinomas is markedly more reduced than those of the TG and TPO genes and that TSH-R expression is significantly reduced in only some carcinomas. They also found positive relationships between the expressional levels of the NIS, TPO, TG and TSH-R genes, which is concordant with our findings. We found positive correlations between the expressions of NIS and TG, and between NIS and PD. Theoretically, the expressions of these thyroid-specific genes would decline according to the thyroid carcinoma dedifferentiation level.
A small number of studies has been performed on the expression of the Glut-1 gene in thyroid carcinoma. Some investigators found increased Glut-1 expression in thyroid cancer [4, 6], and Schonberger et al. [5] showed that Glut-1 protein expression in the cell membrane is closely related to the thyroid neoplasm grade of malignancy. In the present study, we found Glut-1 expression in all papillary cancers except one. Moreover, the expression of the Glut-1 gene was found to be low in the relatively well-differentiated group compared to the relatively less differentiated group, and a negative correlation was observed between the expression of Glut-1 and NIS. These findings explain the well-recognized phenomenon of “flip-flop” in FDG PET and radioiodine whole-body scans and its dependence on the differentiation of thyroid cancer [27, 28]. Clinically, it is well known that FDG PET imaging has an advantage in the detection of recurrent or metastatic cancer in patients who have negative radioiodine scans and that PET may fail to localize tumor sites in patients with a differentiated thyroid cancer that retains iodine metabolic activity [7, 8].
In this study, papillary carcinomas were allocated to the “relatively well-differentiated” or “relatively less differentiated” groups. Classic and papillary carcinoma variants have been found to be related to prognosis. A few like the tall cell and dedifferentiated variants are recognized to be aggressive as compared with classical differentiated papillary carcinomas [29, 30]. In addition, relatively poor prognosis of histological subtypes, such as the oxyphilic and diffuse sclerosing types, has been suggested [31, 32]. Our relatively less differentiated group contained these subtypes associated with a poor prognosis, as both overall architecture in low-power microscopic fields and cytologic features were considered when allocating cases to groups. The high expression of Glut-1 and the low expression of NIS observed in the relatively less differentiated group in the present study support the validity of the pathologic classification used.
Conclusion
Whereas, the NIS and PD genes were highly expressed in relatively well-differentiated papillary thyroid carcinomas, Glut-1 gene expression was highly expressed in relatively less differentiated thyroid carcinomas. These findings provide a molecular rationale for the management of papillary carcinoma, especially with respect to the selection of FDG PET or radioiodine whole-body scans and I-131-based therapy.
Acknowledgments
This study was supported by the Seoul National University Hospital Research Fund (grant no. 03-2004-013-0) and a grant from the Korean Health Technology R&D Project, Ministry of Health, Welfare & Family Affairs, Republic of Korea (A070001), and a grant from Korea University (grant no. K1132631 and K0714991).
Conflict of Interest
Sungeun Kim, June-Key Chung, Hae-Sook Min, Joo-Hyun Kang, Do Joon Park, Jae Min Jeong, Dong Soo Lee, Sung-Hwae Park, Bo Youn Cho, Sinae Lee and Myung Chul Lee declare no conflict of interest.
References
- 1.Taurog A. Hormone synthesis. In: Braverman LE, Utigen RD eds. The thyroid: a fundamental and clinical text. Philadelphia: Lippincott-Raven; 1996: p. 47–81.
- 2.Ros P, Rossi DL, Acebron A, Santisteban P. Thyroid-specific gene expression in the multi-step process of thyroid carcinogenesis. Biochimie. 1999;81:389–396. doi: 10.1016/S0300-9084(99)80086-8. [DOI] [PubMed] [Google Scholar]
- 3.Chung JK, Lee YJ, Kim C, et al. Mechanism related to [18 F]fluorodeoxyglucose uptake of human colon cancers transplanted in nude mice. J Nucl Med. 1999;40:339–346. [PubMed] [Google Scholar]
- 4.Haber RS, Weiser KR, Pritsker A, Reder I, Burstein DE. Glut-1 glucose transporter expression in benign and malignant thyroid nodules. Thyroid. 1997;7:363–367. doi: 10.1089/thy.1997.7.363. [DOI] [PubMed] [Google Scholar]
- 5.Schonberger J, Ruschoff J, Grimm D, Marienhagen J, Rummele P, Meyringer R, et al. Glucose transporter 1 gene expression is related to thyroid neoplasms is related with an unfavorable prognosis: an immunohistochemical study. Thyroid. 2002;12:747–754. doi: 10.1089/105072502760339307. [DOI] [PubMed] [Google Scholar]
- 6.Matsuzu K, Segade F, Matsuzu U, Carter A, Bowden DW, Perrier ND. Differential expression of glucose trnasporters in normal and pathologic thyroid tissue. Thyroid. 2004;14:806–812. doi: 10.1089/thy.2004.14.806. [DOI] [PubMed] [Google Scholar]
- 7.Chung JK, So Y, Lee JS, Choi CW, Lim SM, Hong SW, et al. Value of FDG PET in papillary thyroid carcinoma with negative 131I whole-body scan. J Nucl Med. 1999;40:986–992. [PubMed] [Google Scholar]
- 8.Wang W, Macapinlac H, Larson SM, Yeh SD, Akhurst T, Finn RD, et al. [18F]-2- fluoro-2-deoxy-D-glucose positron emission tomography localizes residual thyroid cancer in patients with negative diagnostic 131I whole body scans and elevated serum thyrolgobulin levels. J Clin Endocrinol Metab. 1999;84:2291–2302. doi: 10.1210/jcem.84.7.5827. [DOI] [PubMed] [Google Scholar]
- 9.Carrasco N. Iodide transport in the thyroid gland. Biochim Biophys Acta. 1993;1154:65–82. doi: 10.1016/0304-4157(93)90017-I. [DOI] [PubMed] [Google Scholar]
- 10.Chung JK. Sodium iodide symporter: its role in nuclear medicine. J Nucl Med. 2002;43:1188–1200. [PubMed] [Google Scholar]
- 11.Chung JK, Kang JH. Translational research using the sodium iodide symporter in imaging and therapy. Eur J Nucl Med Mol Imaging. 2004;31:799–802. doi: 10.1007/s00259-004-1475-3. [DOI] [PubMed] [Google Scholar]
- 12.Lazar V, Bidart B, Caillou C, Mahe C, Macroix L, Filett S, et al. Expression of the Na+/I- symporter gene in human thyroid tumors: a comparison study with other thyroid specific genes. J Clin Endocrinol Metab. 1999;84:3228–3334. doi: 10.1210/jcem.84.9.5996. [DOI] [PubMed] [Google Scholar]
- 13.Hedinger C, Williams ED, Sobin LH. Histological typing of thyroid tumours. Word Health Organization International Histological Classification of Tumours. Berlin: Springer Verlag; 1988. ISBN 2.
- 14.Ponte P, Ng SY, Engel J, Gunning P, Kedes L. Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA. Nucleic Acids Res. 1984;31:1687–1696. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malthie´ryiry Y, Lissitzky S. Primary structure of human thyroglobulin deduced from the sequence of its 8448-base complementary DNA. Eur J Biochem. 1987;165:491–498. doi: 10.1111/j.1432-1033.1987.tb11466.x. [DOI] [PubMed] [Google Scholar]
- 16.Liebert F, Ruel J, Ludgate M, Swillens S, Alexander N, Vassart ZG, et al. Complete nucleotide sequence of the human thyroperoxidase-microsomal antigen cDNA. Nucleic Acids Res. 1987;15:6735. doi: 10.1093/nar/15.16.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagayama Y, Kaufman KD, Seto P, Rapoport B. Molecular cloning, sequence and functional expression of the cDNA for the human thyrotropin receptor. Biochem Bioph Res Co. 1989;165:1184–1190. doi: 10.1016/0006-291X(89)92727-7. [DOI] [PubMed] [Google Scholar]
- 18.Porra V, Bernier-Valentin F, Trouttet-Masson S, et al. Characterization and semiquantitative analyses of pendrin expressed in normal and tumoral human thyroid tissues. J Clin Endocrinol Metab. 2002;87:1700–1707. doi: 10.1210/jcem.87.4.8372. [DOI] [PubMed] [Google Scholar]
- 19.Robbins J, Merino MJ, Boice JD, Jr, Ron Em Ain KB, Alexander HR, et al. Thyroid cancer: a lethal endocrine neoplasm. Ann Intern Med. 1991;115:133–147. doi: 10.7326/0003-4819-115-2-133. [DOI] [PubMed] [Google Scholar]
- 20.Cartro MR, Bergert ER, Goellner JR, Hay ID, Morris JC. Immunohistochemical analysis of sodium iodide symporter expression in metastatic differentiated thyroid cancer: Correlation with radioiodine uptake. J Clin Endocrinol Metab. 2001;86:5624–5632. doi: 10.1210/jcem.86.11.8048. [DOI] [PubMed] [Google Scholar]
- 21.Min JJ, Chung JK, Lee YJ, Jeong JM, Lee DS, Jang JJ, et al. Relationship between expression of the sodium/iodide symporter and 131I uptake in recurrent lesions of differentiated thyroid carcinoma. Eur J Nucl Med. 2001;28:639–645. doi: 10.1007/s002590100509. [DOI] [PubMed] [Google Scholar]
- 22.Patel A, Jhiang S, Dogra S, Terrell R, Powers PA, Fenton C, et al. Differentiated thyroid carcinoma that express sodium-Iodide symporter have a lower risk of recurrence for children and adolescents. Pediatr Res. 2002;52:737–744. doi: 10.1203/00006450-200211000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Ward LS, Santarosa PL, Granja F, Assumpcao LVM, Savoldi M, Goldman GH. Low expression of sodium iodide symporter identifies aggressive thyroid tumors. Cancer Lett. 2003;200:85–91. doi: 10.1016/S0304-3835(03)00392-6. [DOI] [PubMed] [Google Scholar]
- 24.Gerard AC, Daumerie C, Mestdagh C, Cohy C, De Burbure C, Costagliola S, et al. Correlation between the loss of thyroglobulin iodination and the expression of thyroid-specific proteins involved in iodine metabolism in thyroid carcinomas. J Clin Endocrinol Metab. 2003;88:4977–4983. doi: 10.1210/jc.2003-030586. [DOI] [PubMed] [Google Scholar]
- 25.Arturi F, Russo D, Bidart JM, Scarpelli D, Schlumberger M, Filetti S. Expression pattern of the pendrin and sodium/iodide symporter genes in human thyroid carcinoma cell lines and human thyroid tumors. Eur J Endocrinology. 2001;145:129–135. doi: 10.1530/eje.0.1450129. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka K, Otsuki T, Sono H, Yamamoto Y, Udagawa K, Kunisue H, et al. Semiquantitative comparison of the differentiation markers and sodium iodide symporter messenger ribonucleic acids in papillary thyroid carcinomas using RT-PCR. Eur J Endocrinology. 2000;142:340–346. doi: 10.1530/eje.0.1420340. [DOI] [PubMed] [Google Scholar]
- 27.Feine U, Lietzenmayer R, Hanke JP, Held J, Wohrle H, Muller-Schuenburg W. Fluorine-18-FDG and iodine-131-iodide uptake in thyroid cancer. J Nucl Med. 1996;37:1468–1472. [PubMed] [Google Scholar]
- 28.Grunwald F, Menzel C, Bender H, Palmero H, Willkomm P, Ruhlmann J, et al. Comparison of 18FDG-PET with 131iodine and 99mTc-sestamibi scintigraphy in differentiated thyroid cancer. Thyroid. 1997;7:327–335. doi: 10.1089/thy.1997.7.327. [DOI] [PubMed] [Google Scholar]
- 29.Johnson TL, Lloyd RV, Thompson NW, Beierwaltes WH, Sisson JC. Prognostic implications of the tall cell variant of papillary thyroid carcinoma. Am J Surg Pathol. 1988;12:22–27. doi: 10.1097/00000478-198801000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Mizukami Y, Noguchi M, Michigishi T, Nonomura A, Hashimoto T, Otakes S, et al. Papillary thyroid carcinoma in Kanazawa, Japan: prognostic significance of histological subtypes. Histopathology. 1992;20:243–250. doi: 10.1111/j.1365-2559.1992.tb00963.x. [DOI] [PubMed] [Google Scholar]
- 31.Berho M, Suster S. The oncocytic variant of papillary carcinoma of the thyroid: a clinicopathologic study of 15 cases. Hum Pathol. 1997;28:47–53. doi: 10.1016/S0046-8177(97)90278-1. [DOI] [PubMed] [Google Scholar]
- 32.Albareda M, Puig-Domingo M, Wengrowicz S, Solderila J, Matias-Guiu X, Caballero A, et al. Clinical forms of presentation and evolution of diffuse sclerosing variant of papillary carcinoma and insular variant of follicular carcinoma of the thyroid. Thyroid. 1998;8:385–391. doi: 10.1089/thy.1998.8.385. [DOI] [PubMed] [Google Scholar]