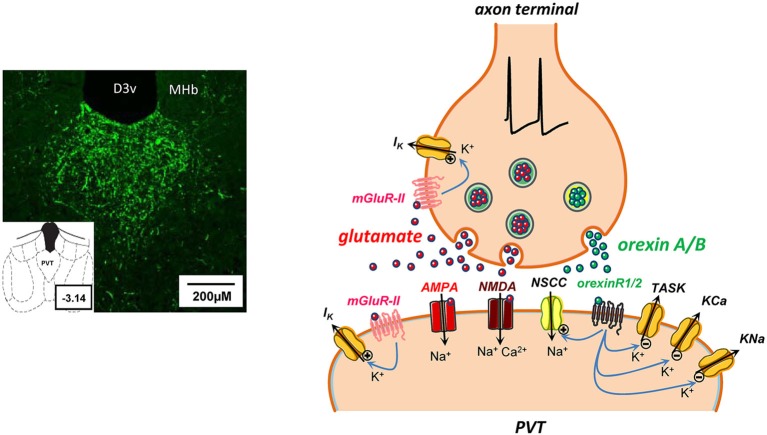
Figure 3.
Some potential consequences of glutamate and orexin co-release at a synapse in PVT. On the left, microphotograph of coronal section from rat brain (bregma ∼ −3.14) reveal a dense distribution of orexin A-immunoreactive fibers in PVT nucleus. Abbreviations: D3v, dorsal 3rd ventricle; MHb, medial habenula. On the right, schematic synapse depicting action potential invasion of an axon terminal in PVT containing storage vesicles for a rapid transmitter (glutamate, red symbols) and a neuropeptide (orexin A or B, green symbols). Presynaptically released glutamate diffuses across the synaptic cleft to act at postsynaptic ionotropic AMPA and NMDA receptors, promoting cation influx and induction of rapid excitatory postsynaptic currents. In addition, glutamate release may potentially activate metabotropic group II (mGluR-II) receptors to open pre- and/or postsynaptic K+ channels (Hermes and Renaud, 2011). Activity-dependent co-release of orexins and activation of metabotropic orexin receptors (orexinR1/2) may have several postsynaptic actions that collectively result in enhanced neuronal excitability by: (a) opening of nonselective cation channels (NSCC; Kolaj et al., 2007); (b) closing of K+ channels, including two-pore-domain TASK-like channels that are constitutively active at rest (Doroshenko and Renaud, 2009); and (c) suppression of KCa and KNa channels underlying spike train induced sAHPs (Zhang et al., 2010).