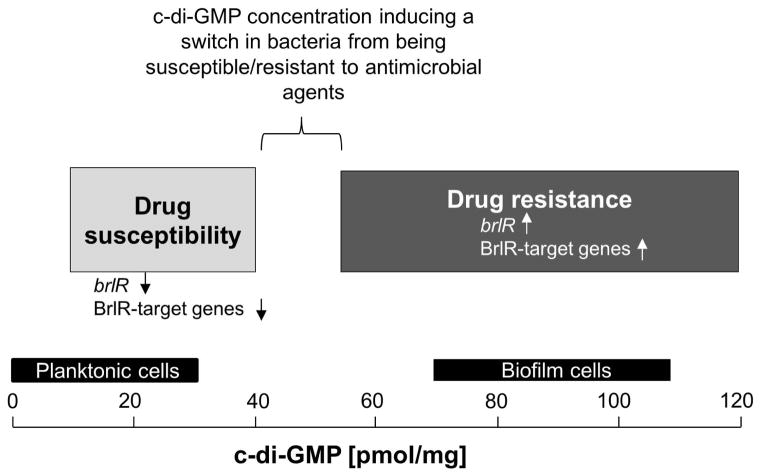
Figure 9. Model for the role of c-di-GMP levels rather than the mode of growth in the brlR expression, BrlR function, and resistance/susceptibility of P. aeruginosa cells to antimicrobial agents.
Based on our findings, the level of c-di-GMP is crucial for the susceptible-resistance switch by P. aeruginosa cells (see also “?” in Fig. 1). P. aeruginosa cells harboring ≤40 pmol/mg c-di-GMP are more susceptible to antimicrobial agents than P. aeruginosa cells harboring ≥55 pmol/mg c-di-GMP. Susceptibility was found to be independent of whether P. aeruginosa was grown planktonically or as biofilm. Light Grey bar, range of c-di-GMP levels determined here to correlate with P. aeruginosa cells being susceptible to antimicrobial agents. Low c-di-GMP levels correlated with significantly reduced or absent brlR expression and reduced DNA binding capability by BrlR. Dark Grey bar, range of c-di-GMP levels determined here to correlate with P. aeruginosa cells being resistant to antimicrobial agents. Elevated c-di-GMP levels correlated with significantly increased brlR expression and BrlR being able to bind to its target promoter DNA. BrlR target genes include mexAB-OprM and mexEF-OprN (Liao et al., 2013). Black bars indicate the range of intracellular c-di-GMP levels previously reported to be present in P. aeruginosa cells grown planktonically and as biofilm (Basu Roy et al., 2012, Barraud et al., 2009). BrlR target genes include the multidrug efflux pump genes mexAB-OprM and mexEF-OprN (Liao et al., 2013).