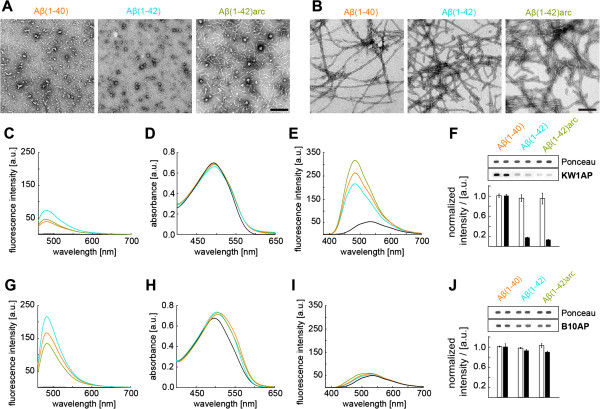
Figure 3.
B10 and KW1 binding to different Aβ structures. (A-B) TEM images of intermediates (A) and amyloid fibrils (B) of the different Aβ variants as indicated. Scale bar represent 200 nm. Intermediates and fibrils are further characterized by interactions with Congo red (C, G), Thioflavin T (ThT, D, H) and 8-anilinonaphthalene-1-sulfonate dyes (ANS, E, I). Measurements with fibrils were carried out in 50 mM sodium borate, pH 9.0. Reactions with intermediates contained 10% HFIP in water as the base solution. (F) Spot blot data of alkaline phosphatase (AP) coupled KW1 binding to intermediates. Densitometric quantifications and representative raw data stained with either Ponceau (loading control, white bars) or KW1AP (black bars). Aβ(1–40) Ponceau and KW1AP signals have been set to 100%. Error bars show standard deviation (n = 2–3). (G-I) Congo red, ThT and ANS binding of fibrils in 50 mM sodium borate buffer, pH 9.0. (J) Spot blot data of AP coupled B10 binding to fibrils. Densitometric quantifications and representative raw data shown. All panels show the same colour coding: black: solution without Aβ; ochre: Aβ(1–40); turquoise: Aβ(1–42); green: Aβ(1–42)arc.