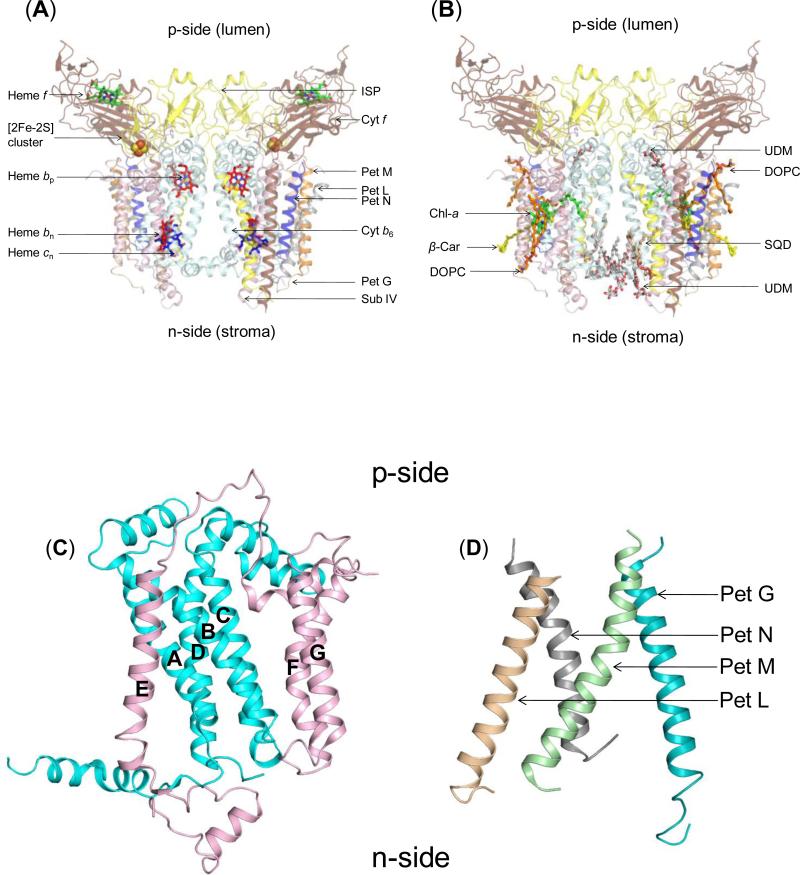
Figure 2. Structure of dimeric b6f complex from M.laminosus (PDB ID 2E74).
(240,000 MW; per monomer, 8 subunits/7 prosthetic groups; 8 lipids) Subunit organization and lipid binding sites. (A) View along membrane plane showing the positions of the 8 subunits. Color code: cytochrome f (pet A), yellow; cytochrome b6 (pet B), cyan); Rieske [2Fe-2S] protein (Pet C), orange; subunit IV/Pet D (pink), Pet G (teal), Pet L (light brown), Pet M (green) and Pet N (gray). (B) Side view of M. laminosus b6f complex showing bound lipids, detergents and pigments. (C) Polytopic core of the b6f complex. Cyt b6 (4 TMH, cyan) and subunit IV (3 TMH, pink) form the core of the b6f monomer. The cytb6TMH form a four helix bundle. SubIV is organized around the bundle as a bipartite structure, with the E-helix separated from the F and G TMH. (D) Peripheral four helix bundle formed by the small Pet subunits, Pet G, L, M and N. In addition, including ferredoxin-NADP+-reductase (FNR), which may mediate electron transfer from PSI to cyt b6f. (Fig. 1) In addition, there are four soluble subunits that associate with purified b6f complex from plant or algal sources, but have not been seen in the crystal structures and have presumably dissociated and been lost during crystallization: the Pet P polypeptide seen in cyanobacteria, the light-harvesting LHCII chlorophyll protein kinase Stt7-STN7, the correlated phosphatase, and the PetO nuclear-encoded phosphorylatable subunit,.