Abstract
OBJECTIVE
To compare effects of combinations of standard and intensive treatment of glycemia and either blood pressure (BP) or lipids in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial.
RESEARCH DESIGN AND METHODS
ACCORD enrolled 10,251 type 2 diabetes patients aged 40–79 years at high risk for cardiovascular disease (CVD) events. Participants were randomly assigned to hemoglobin A1c goals of <6.0% (<42 mmol/mol; intensive glycemia) or 7.0–7.9% (53–63 mmol/mol; standard glycemia) and then randomized a second time to either 1) systolic BP goals of <120 mmHg (intensive BP) or <140 mmHg (standard BP) or 2) simvastatin plus fenofibrate (intensive lipid) or simvastatin plus placebo (standard lipid). Proportional hazards models were used to assess combinations of treatment assignments on the composite primary (deaths due to CVD, nonfatal myocardial infarction [MI], and nonfatal stroke) and secondary outcomes.
RESULTS
In the BP trial, risk of the primary outcome was lower in the groups intensively treated for glycemia (hazard ratio [HR] 0.67; 95% CI 0.50–0.91), BP (HR 0.74; 95% CI 0.55–1.00), or both (HR 0.71; 95% CI 0.52–0.96) compared with combined standard BP and glycemia treatment. For secondary outcomes, MI was significantly reduced by intensive glycemia treatment and stroke by intensive BP treatment; most other HRs were neutral or favored intensive treatment groups. In the lipid trial, the general pattern of results showed no evidence of benefit of intensive regimens (whether single or combined) compared with combined standard lipid and glycemia treatment. The mortality HR was 1.33 (95% CI 1.02–1.74) in the standard lipid/intensive glycemia group compared with the standard lipid/standard glycemia group.
CONCLUSIONS
In the ACCORD BP trial, compared with combined standard treatment, intensive BP or intensive glycemia treatment alone improved major CVD outcomes, without additional benefit from combining the two. In the ACCORD lipid trial, neither intensive lipid nor glycemia treatment produced an overall benefit, but intensive glycemia treatment increased mortality.
Introduction
Cardiovascular disease (CVD) is the most common cause of death and disability in patients with type 2 diabetes mellitus, and dysglycemia, high blood pressure (BP), and dyslipidemia mediate much of this increased CVD risk (1). Treatment trials focused on single risk factor modification have shown that lowering glucose, BP, and lipids substantially reduces CVD events and deaths in patients with type 2 diabetes (2–4). The Steno-2 Study showed a major reduction in CVD events and mortality with multifactorial intensive treatment (5,6). When the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial was designed, the study questions focused on the separate effectiveness of three distinct single-factor interventions targeting near-normal levels of hemoglobin A1c (HbA1c) versus standard levels, near-normal levels of systolic BP versus standard levels, and combined lipid-lowering therapy with a fibrate and an hydroxymethylglutaryl CoA reductase inhibitor (statin) versus monotherapy with a statin (7–10).
Assessment of the effectiveness of combined versus single intensive interventions was not the primary objective of ACCORD. However, the factorial study design permits these planned analyses, and their potential relevance has only increased given the somewhat unexpected pattern of findings of the ACCORD trials (11–14). None of the three intensive intervention strategies had a significant beneficial main effect on the primary outcome, a composite of cardiovascular death, nonfatal myocardial infarction (MI), and nonfatal stroke (major CVD). All-cause mortality and CVD mortality were increased by the intensive glycemia intervention and not altered by intensive treatment of BP or an intensive lipid treatment strategy. At the same time, the risk of nonfatal MI was reduced by intensive treatment of glycemia, and risk of fatal and nonfatal stroke was reduced by intensive treatment of BP.
In previously published analyses, a modest interaction was observed for the outcome of total mortality between the intensive glycemia and BP interventions (P = 0.03); the group that received both intensive interventions had the highest mortality, while the groups receiving either or both standard interventions had lower mortality (Supplemental Fig. 4 in 11). No significant interaction was found for the primary composite outcome of major CVD, and no interactions were found for either outcome between the glycemia and lipid interventions. However, intensive glycemia assignment was associated with a nonsignificant 26–33% elevated risk of mortality regardless of lipid treatment assignment.
The purpose of this article is to explore these treatment combinations in more depth by examining outcomes separately in the lipid and BP trials and by examining outcomes separately for the intensive and standard glycemia participants within those trials. In the BP trial, we compare standard treatment of glycemia and BP with both single and joint intensive treatment of glycemia and BP. In the lipid trial, we conduct a parallel comparison of standard glycemia and lipid treatment with both single and joint intensive interventions. This analytic strategy was not prespecified but was developed before the data presented here were analyzed. The goal was to determine whether macrovascular, microvascular, or mortality outcomes differed in any of the three intensively treated groups in each trial compared with the group that received standard treatment.
Research Design and Methods
The rationale, design, methods, and major outcomes of the ACCORD trial have been published previously (7–10,12–15). In brief, ACCORD was a double two-by-two factorial trial designed to test whether, compared with standard treatment, more intensive control of glycemia, BP, or dyslipidemia might reduce risk of cardiovascular death, nonfatal MI, and nonfatal stroke (major CVD). Men and women with type 2 diabetes aged 40 to 79 years and with HbA1c ≥7.5% (≥58 mmol/mol) and who had prior evidence of CVD or multiple cardiovascular risk factors were recruited in 77 clinics across the U.S. and Canada from January 2001 to October 2005. All participants were required to qualify for the glycemia trial and at least one of the nested trials (BP or lipid). In the glycemia trial, participants were randomized to HbA1c goals of <6.0% (<42 mmol/mol; intensive) or 7.0–7.9% (53–63 mmol/mol; standard). In the BP trial, participants were randomized to systolic BP goals of <120 mmHg (intensive) or <140 mmHg (standard). In the lipid trial, participants were randomized in a masked fashion to combined therapy with fenofibrate plus simvastatin (intensive) or placebo plus simvastatin (standard).
The glycemia trial was terminated early due to an observed 22% greater relative risk of death in the intensively treated participants (12). Participants were informed of the decision to terminate the glucose-lowering comparison on 5 February 2008, after a mean treatment period of 3.7 years. From this transition date onward, the intensity of glycemia interventions in the intensive group was reduced to that of the standard group and HbA1c targets were changed from <6% (<42 mmol/mol) to between 7 and 7.9% (53–63 mmol/mol). As participants had also been allocated to either the BP trial or the lipid trial, they continued to be followed per protocol either every 2 or 4 months until the originally planned trial end (June 2009). Thus clinical outcomes continued to be collected and blindly adjudicated centrally for an additional 17 months.
The effects of the glycemia intervention during a mean of 3.5 years that provided the basis for the Data Safety Monitoring Board (DSMB) recommendation were previously reported (12) and were updated to reflect the effect of the intervention the additional 0.2 years (i.e., until 5 February 2008) when intensive glycemia participants were informed of the change in glycemia management (11). This article reports the effects of the glycemia, BP, and lipid interventions over of a mean of 3.7 years of follow-up using an intention-to-treat analysis.
The primary end point for ACCORD was major CVD events, defined as the composite of deaths due to CVD, nonfatal MI, and nonfatal stroke. This article will also report the following secondary outcomes: total mortality, CVD deaths, any MI, any stroke, an expanded macrovascular outcome (including major fatal and nonfatal CVD events in the primary outcome, revascularizations, and heart failure hospitalizations), and the two protocol-specified microvascular disease composite outcomes (15). The first microvascular disease composite combined advanced kidney and eye disease and was intended to approximate the primary microvascular outcome of the UK Prospective Diabetes Study (16); the second added peripheral neuropathy to that outcome.
Variables are described using the mean (SD) or median (interquartile range) for continuous measures and proportions for categorical variables. The use of medications from various classes is described based on the last visit for each participant prior to 5 February 2008, when the intensive glycemia intervention was stopped. Mean HbA1c, BP, and lipid levels at the last visit prior to stopping the glycemia intervention are reported for each of the four combination of treatment assignments in both the BP and lipid trials. Differences in event rates between randomized groups were tested using proportional hazards regression analysis adjusting for the design factors of clinical center network, baseline history of CVD, and BP trial or lipid trial assignment. All analyses were performed using SAS version 9. In keeping with other ACCORD secondary analyses, adjustment for multiple comparisons was not performed.
ACCORD was sponsored by the National Heart, Lung, and Blood Institute (NHLBI) with support from other National Institutes of Health (NIH) institutes, and the protocol was reviewed, approved in advance, and monitored by an NHLBI DSMB and by ethics committees at each center. All participants provided written informed consent. All authors vouch for the accuracy and completeness of the reported data.
Results
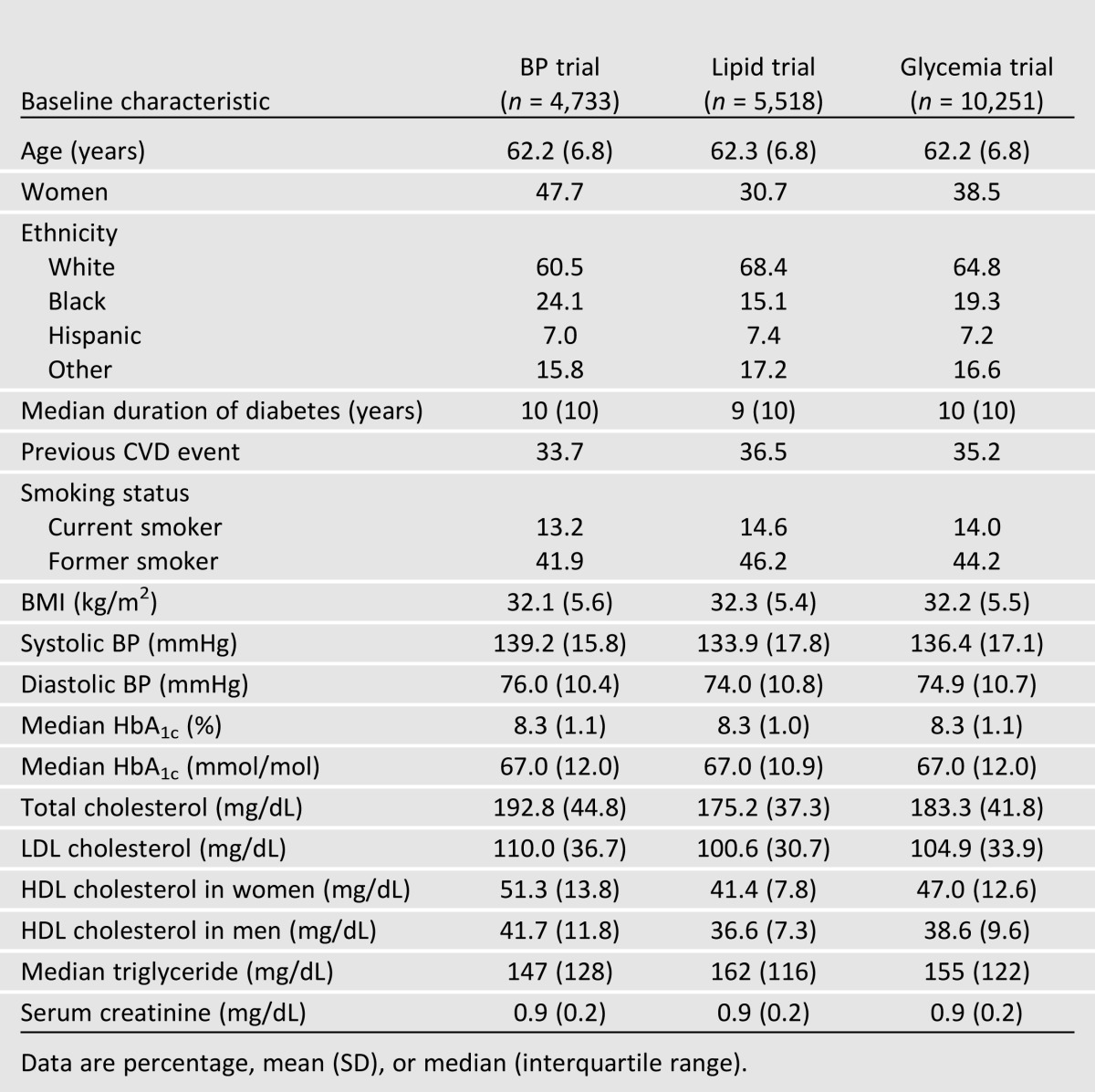
The allocation of participants and the completeness of follow-up in the ACCORD trial have been reported previously (12–14). Characteristics of the 10,251 participants by trial are shown in Table 1. Overall, participants included an ethnically diverse group of middle-aged and older men and women with poorly controlled type 2 diabetes of long duration. Mean BMI at randomization was 32.2 kg/m2, and 35.2% had one or more major cardiovascular events prior to randomization. Although all participants qualified for the glycemia trial, the inclusion and exclusion criteria for the BP and lipid trials differed by design (8–10). Participants in the BP trial and the lipid trial differed on several characteristics: BP trial participants were more likely to be black, to be female, and to have higher BP levels. They also had lower triglyceride levels and higher HDL and LDL cholesterol concentrations. Lipid trial participants were more likely to have smoked and to have had previous CVD events, had lower BP, had higher triglyceride levels, and had lower HDL and LDL cholesterol concentrations.
Table 1.
Baseline characteristics of ACCORD trial participants
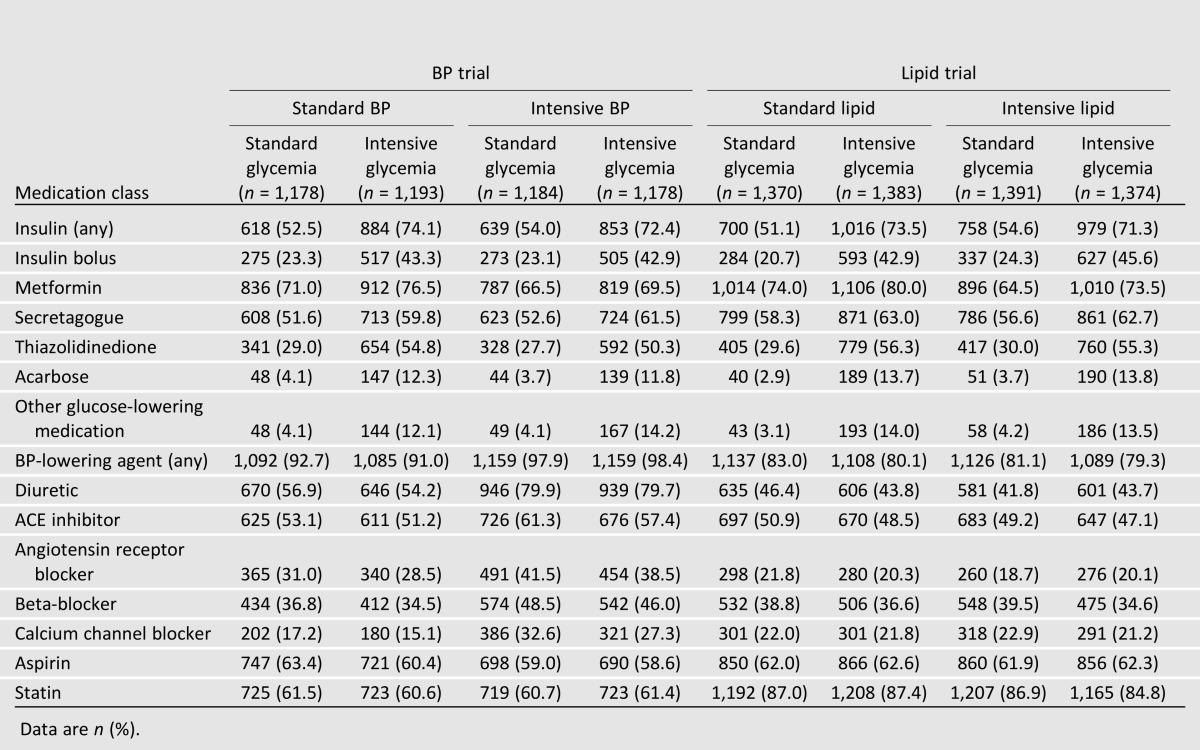
The treatments prescribed to participants are shown by treatment group in Table 2. All glucose-lowering medications were used more commonly and at higher mean doses in the intensive glycemia group, but the largest differences were for insulin use (any and bolus) and thiazolidinediones. Similarly, all major BP-lowering medication classes were used more commonly in the intensive BP group, with the largest difference for diuretics. BP medications were used similarly across lipid trial groups. Aspirin use was similar across groups. Statins were used similarly in nearly in all lipid trial participants by design and were also used similarly but at lower levels in BP trial participants.
Table 2.
Prevalence of medication use by participants in various treatment groups of the ACCORD BP and lipid trial at the last visit prior to stopping the intensive glycemia intervention
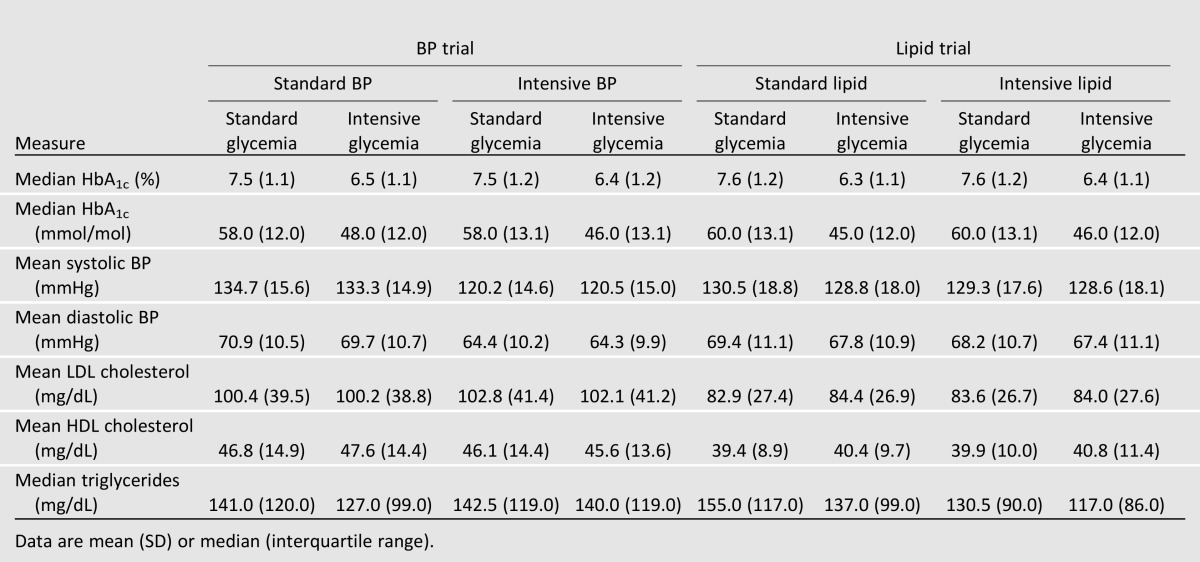
In the BP trial, mean HbA1c levels at the last visit were similar in standard and intensive glycemia participants regardless of treatment assignment to standard or intensive BP intervention (Table 3). Parallel analyses in the lipid trial participants showed similar findings. Mean BP levels were 1–2 mmHg lower in intensive glycemia group participants than in standard glycemia group participants in the lipid trial and in the standard BP groups of the BP trial. LDL cholesterol levels were similar across all treatment assignments in both trials. HDL cholesterol levels were highest relative to the other treatment assignments in the standard BP/intensive glycemia group in the BP trial and in the intensive lipid/intensive glycemia group in the lipid trial. Triglyceride levels were lowest in the intensive glycemia and intensive lipid treatment groups.
Table 3.
HbA1c, BP, and lipids at the last visit prior to stopping the intensive glycemia intervention
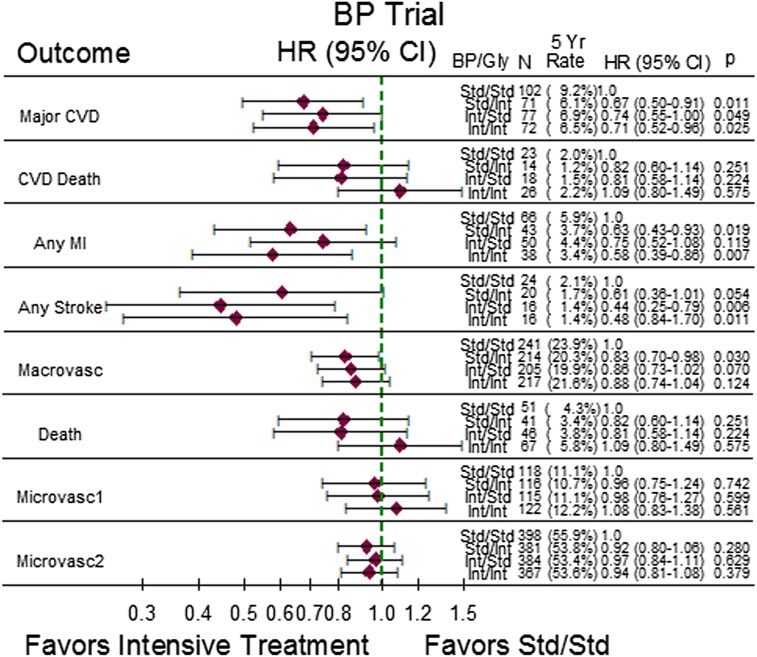
The hazard ratio (HR), corresponding 95% CI, and 5-year event rates for the study outcomes are shown in Fig. 1 for the BP trial and in Fig. 2 for the lipid trial. In both figures, the least intensively treated group (standard BP/standard glycemia group or standard lipid/standard glycemia group) serves as the reference group. In the BP trial, most of the point estimates of the HRs were neutral or favored the more intensively treated groups. All three more intensively treated groups in the BP trial experienced significantly lower rates of the primary outcome, major CVD, with HRs ranging from 0.67 to 0.74 when compared with the standard BP/standard glycemia group. Both of the more intensively treated glycemia groups experienced lower rates of MI, while both of the more intensively treated BP groups experienced lower rates of stroke when compared with the standard BP/standard glycemia group. The expanded macrovascular end point favored all of the more intensively treated groups, but only the standard BP/intensive glycemia group had a significantly lower event rate than the standard BP/standard glycemia reference group (HR 0.83; 95% CI 0.70–0.98). There was no statistical evidence of benefit or harm regarding total mortality, CVD mortality, or microvascular disease for any of the more intensively treated groups. Of particular note, while the intensive BP/intensive glycemia group showed evidence of benefit regarding major CVD, any MI, and any stroke compared with the standard BP/standard glycemia group, the benefits were not different from those observed for the single-factor intensive regimens.
Figure 1.
Five-year event rates, HR, and corresponding 95% CI for comparisons of the three more intensively treated groups to the standard BP-lowering/standard glucose-lowering treatment group in the ACCORD BP trial. P values are for pairwise comparisons of more intensively treated groups with the standard/standard group. BP, blood pressure; Gly, glycemia; std, standard; int, intensive; macrovasc, macrovascular end point; microvasc, microvascular end point.
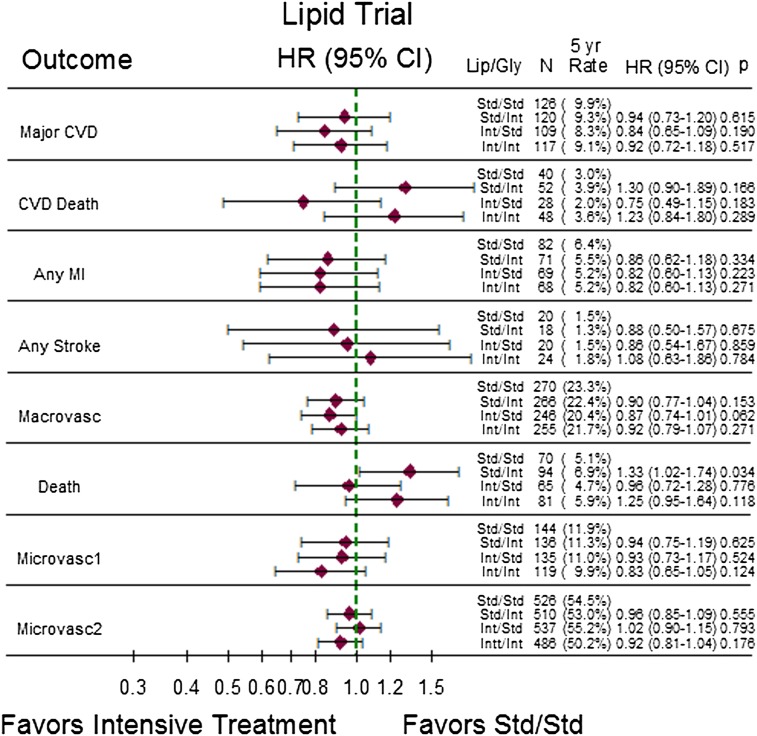
Figure 2.
Five-year event rates, HR, and corresponding 95% CI for comparisons of the three more intensively treated groups to the fibrate placebo/standard glucose-lowering treatment group in the ACCORD lipid trial. P values are for pairwise comparisons of more intensively treated groups with the standard (placebo)/standard group. Gly, glycemia; Lip, lipid; std, standard; int, intensive; macrovasc, macrovascular end point; microvasc, microvascular end point.
In the lipid trial, the general pattern of results showed no evidence of benefit of intensive regimens (whether single factor or combined) relative to the standard lipid/standard glycemia group. Mortality was 33% higher in the standard lipid/intensive glycemia group than in the standard lipid/standard glycemia group (HR 1.33; 95% CI 1.02–1.74), and total and CVD mortality were nonsignificantly higher in the intensive glycemia group relative to the standard lipid/standard glycemia group regardless of lipid treatment assignment.
Conclusions
Although none of the individual intensive interventions reduced the ACCORD trial primary outcome of major CVD, these current planned analyses of the effects of single and combined intensive intervention regimens on major macrovascular outcomes in the ACCORD trial provide a somewhat different view of the results than those published to date. First, compared with the standard BP/standard glycemia group, the intensive BP/intensive glycemia, intensive BP/standard glycemia, and standard BP/intensive glycemia groups all showed benefit for reducing the risk of major CVD in the BP trial. However, the intensive BP/intensive glycemia group showed no evidence of incremental benefit compared with either single intensive intervention. Second, in the BP trial, MI was significantly reduced in the intensive glycemia treatment groups and stroke in the intensive BP treatment groups. Third, compared with the standard lipid/standard glycemia group, there was no evidence of benefit of either the intensive lipid or intensive glycemia intervention in the lipid trial, and there was increased mortality in the standard lipid/intensive glycemia group. None of the intensive interventions, singly or in combination, affected the risk of advanced microvascular outcomes, which is consistent with previously published findings (15,17).
These results raise several questions. Why did the intensive BP and intensive glycemia interventions fail to show additive effects in the BP trial? For major CVD, MI, stroke, and macrovascular disease, one or both of the single-factor intensive regimens was at least as effective in reducing risk as was the combined intensive regimen. Might this finding provide evidence of a “floor effect,” i.e., a diminishing ability of intensive treatments to lower risk further when one risk factor is reduced to near-normal levels and the other risk factor is reasonably well controlled? While this possibility is speculative, the results of the AIM HIGH (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes) trial provide additional evidence of some pertinence (18). In this trial, in the setting of very well-controlled cholesterol (using a moderate-dose statin), further lowering of LDL cholesterol by the addition of niacin had no further impact on risk of CVD, despite achieving better HDL cholesterol and triglyceride levels. The results of AIM HIGH contrast with results of the Coronary Drug Project, conducted in the prestatin era, which demonstrated a cardioprotective effect of niacin alone (19). If the reason for that discrepancy is the standard use of statins in AIM HIGH, then it seems possible that the substantial use of aspirin in over 60% in both trials, and statins in over 60% in the ACCORD BP trial and over 85% in the ACCORD lipid trial, may have reduced the ability of the intensive interventions in ACCORD to further reduce CVD outcomes. Similarly, in studies designed to show safety of newer glucose-lowering drugs, the new agent is typically added to existing therapy in patients receiving high-quality background therapy, many of whom also have well-controlled glycemia. In the context of the ACCORD results, perhaps it is not surprising that such studies have failed to show CVD benefit (20,21).
Why did the intensive glycemia intervention appear to reduce the risk for major CVD in the BP trial but not in the lipid trial, and why did it appear to increase mortality in the lipid trial but not in the BP trial? Unlike a 3 × 3 design, which would have required a much larger sample size, the double 2 × 2 factorial study design did not permit a comparison of various combinations of all three interventions. Given differences in eligibility criteria and underlying risk for CVD events for the participants in the two trials, a simple comparison of the event rates for the intensive glycemia groups in the BP and lipid trials could be misleading. Although the characteristics of the participants in the two trials differed somewhat, there was substantial overlap, and previous subgroup analyses from ACCORD do not point to any measured participant characteristics or drug treatments that might explain the difference in the estimated effect of the intensive glycemia intervention on CVD between the trials. There may have been more subclinical CVD in the lipid trial, an unmeasured characteristic that could have influenced the risk of subsequent major CVD. In previous subgroup analyses, intensive glycemia group participants with a CVD event before randomization had a higher risk of major CVD during the trial compared with participants with no prior history of CVD (12). It is also possible that the greater use of statins in the lipid trial reduced the ability to detect the glycemia treatment effect observed in the BP trial, although this seems unlikely given that event rates in the reference group in the lipid trial were similar or higher than in the BP trial. Finally, given the lack of an overall effect of the glycemia intervention in ACCORD, chance cannot be excluded as the basis for the difference between the trials.
In the Steno-2 Study, an intensive multifactorial intervention that included targeting glycemia, BP, lipids, and aspirin use reduced the risk of CVD by ∼50% in patients with diabetes over 7.8 years of follow-up (6). How can the results of Steno-2 and ACCORD be reconciled? First, it is important to note that the intensive levels of risk factor control achieved in Steno-2 approximate the standard levels of control achieved in ACCORD. It is possible that ACCORD was operating on a much flatter part of the risk curve. That is, it is possible that the benefits of going from fair risk factor control (Steno-2 standard group) to moderate risk factor control (Steno-2 intensive group) exceed the benefits of going from moderate risk factor control (ACCORD standard groups) to intensive risk factor control (ACCORD intensive groups). Second, it is not possible to determine from Steno-2 whether the entire multifactor intervention was needed, which interventions provided most of the benefit, or whether an effect almost as great might have been achieved with a less comprehensive approach. The intensive intervention in Steno-2 included many lifestyle interventions that were recommended as background therapy for both randomized groups in ACCORD. It is notable that the Steno-2 Study achieved positive outcomes with a mean HbA1c of 7.8% (62 mmol/mol) in the intensive group, while mean systolic BP was ∼132 mmHg and mean LDL was ∼80 mg/dL. Given the two-group design of the trial, it is impossible to disentangle the individual treatment effects in Steno-2.
Several factors constrain the interpretation of the data presented here. First, although these analyses were planned in the ACCORD study, they are secondary analyses and should mainly be viewed as exploratory. Second, because we analyzed results across individual cells of a factorial design with shorter follow-up than originally intended, our power to detect meaningful differences and interactions between groups was reduced. On the other hand, the observed P values must be considered in light of the 48 reported comparisons. By chance alone, two or three significant results would be expected at P = 0.05, fewer than the eight we observed. Third, the observed results may apply only to ACCORD-like patients; extrapolation to other patients, especially to younger or healthier patients with shorter duration of diabetes, must await the results of additional trials. The recently completed Secondary Prevention of Small Subcortical Strokes (SPS3) trial provides some additional evidence of stroke reduction by intensive BP control in patients with recent lacunar stroke. SPS3 compared a systolic BP target of 130–149 mmHg with a target <130 mmHg and achieved a separation of 11 mmHg between the groups. There was a nonsignificantly lower risk of the primary outcome of recurrent stroke in the group treated to the lower BP target (HR 0.81; 95% CI 0.64–1.03; P = 0.08) and a significant reduction in hemorrhagic stroke (HR 0.37; 95% CI 0.15–0.95; P = 0.03) (22). In addition, the ongoing Systolic Blood Pressure Intervention Trial (SPRINT) in is testing a similar intensive BP intervention in a nondiabetic population (https://www.sprinttrial.org/public/dspHome.cfm; accessed 20 June 2013.)
We now summarize the results of the current secondary analyses, place them in the context of the previously published primary analyses, and discuss implications for clinical practice. In diabetes patients resembling those in the ACCORD lipid trial, addition of fenofibrate to a statin, intensive glycemia control, or the combination did not prevent major CVD or other CVD outcomes better than standard glycemia control with statin use. These results are consistent with the primary analyses, except that the previously observed increase in mortality associated with intensive glycemia control seems limited to this subset of ACCORD participants. These secondary analyses newly suggest that in diabetes patients resembling those in the ACCORD BP trial, either intensive BP or glycemia control reduces major CVD compared with combined standard treatment, but the combination was no better than the individual intensive interventions. The current results are consistent with the previously published primary analyses in that intensive glycemia control reduces MI while intensive BP control reduces stroke, although the glycemia findings are limited to the subset of ACCORD participants in the BP trial. In this subset, there was no evidence of increased mortality with either intensive intervention.
Both the intensive BP control and intensive glycemia control strategies require more medications and more visits than the standard control strategies. Participants in both intensive treatment groups also experienced significantly higher rates of adverse effects that were attributed to treatment. These included more weight gain and serious hypoglycemia in the intensive glycemia group and more hypotension, syncope, bradycardia/arrhythmia, and hyperkalemia in the intensive BP group (12–14). Nevertheless, it may be easier and safer to achieve near-normal systolic BP and to use statins than to achieve near-normal levels of HbA1c with the multidrug glucose-lowering regimens that were used in ACCORD. These and previous ACCORD analyses suggest that there is a potential for increased mortality with intensive glycemia treatment in a subset of patients who are difficult to identify. There is no corresponding evidence of an increased mortality risk with intensive BP treatment. A recent meta-analysis of treatment trials in people with type 2 diabetes who had systolic BP ≤135 mmHg in the intensive BP group and ≤140 mmHg in the standard BP group found a 10% (95% CI 2–17%) reduction in total mortality (23). Several trials, including the ACCORD BP trial, with intensive group systolic BP <120 were included in the meta-analysis, and none found increased mortality in the <120 mmHg group.
In patients with longstanding type 2 diabetes and high CVD risk resembling those in the ACCORD lipid trial, these analyses do not support adding fenofibrate to a statin, intensive glycemia control, or the combination. In patients similar to those enrolled in the ACCORD BP trial, these analyses provide limited support for intensive BP control and even more limited support for intensive glycemia control to reduce the risk of major CVD. Any kind of treatment intensification requires more visits and medications and exposure to adverse effects, but intensifying BP control in patients with diabetes may be less risky than intensifying glycemia control, which reduced MI but was associated with increased mortality in some patients. Thus intensive BP control may be reasonable in certain motivated patients who have been educated about the added treatment burden, cost, and risk of side effects and want to further lower their risk of stroke beyond what can be achieved through standard care. There may be other patients for whom intensive glycemia control may be beneficial to further reduce their risk of MI. From a clinical point of view, the results presented here confirm the great importance of tailoring treatment goals and treatment intensity to specific subgroups of patients. The choice of whether to pursue either intensive BP control or intensive glycemia control using multidrug regimens will require shared decision making between the physician and patient about their individual medical profiles, goals, and preferences.
Article Information
Acknowledgments. The authors thank Denise Simons-Morton for her leadership of ACCORD at the NHLBI.
Funding. This work was supported by the NHLBI (contracts N01-HC-95178, N01-HC-95179, N01-HC-95180, N01-HC-95181, N01-HC-95182, N01-HC-95183, N01-HC-95184, IAA-Y1-HC-9035, and IAA-Y1-HC-1010) and partially supported by the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Eye Institute and by General Clinical Research Centers at many sites. Substudies within the ACCORD trial on cost-effectiveness and health-related quality of life were supported by the Centers for Disease Control and Prevention. All authors were supported by NIH grant funds for the ACCORD trial during the study. K.L.M. has NIH studies with funds going to her institution and assumes all responsibility for the work as a whole. W.C.C. reports grants from NHLBI and NIH during the conduct of the study. The following companies provided study medications, equipment, or supplies: Abbott Laboratories, Amylin Pharmaceuticals, AstraZeneca, Bayer HealthCare, Closer Healthcare, GlaxoSmithKline, King Pharmaceuticals, Merck, Novartis, Novo Nordisk, Omron Healthcare, Sanofi, and Schering-Plough.
Duality of Interest. J.B.B. is an investigator and/or consultant without any direct financial benefit to him under contracts between his employer and the following companies: Abbott, Amylin, Andromeda, AstraZeneca, BD Research Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Catabasis, Cebix, Diartis, Elcelyx, Eli Lilly, Exsulin, Genentech, GI Dynamics, GlaxoSmithKline, Halozyme, Hoffmann-La Roche, Johnson & Johnson, LipoScience, Medtronic, Merck, Metabolic Solutions Development Company, Metabolon, Novan, Novartis, Novella Clinical, Novo Nordisk, Orexigen, Osiris, Pfizer, Rhythm, Sanofi, Spherix, Takeda, Tolerex, Trans Pharma, Veritas, and Verva. R.M.C. is affiliated with a nonprofit entity that received a research grant from Novo Nordisk for his work on a clinical trial. W.C.C. reports personal fees from Takeda, personal fees from AstraZeneca, grants and personal fees from Merck, personal fees from Omron, personal fees from Daiichi Sankyo, and personal fees from Novartis outside the submitted work. H.C.G. has received consulting fees from Sanofi, Lilly, Novo Nordisk, Bristol-Myers Squibb, Roche, AstraZeneca, Novartis, and GlaxoSmithKline; lecture fees from Sanofi; and support for research or continuing education through his institution from Sanofi, Lilly, Merck, Novo Nordisk, Boehringer Ingelheim, Bristol-Myers Squibb, and AstraZeneca. R.H.G. reports personal fees and travel funds from International Atherosclerosis Society Official Symposium Lecture, other funding from AleCardio, and a grant to his institution from Roche outside the submitted work. M.C.R. has received honoraria for consulting and/or speaking from Amylin, Eli Lilly, Elcelyx, Sanofi, and Valeritas and research grant support through his institution from Amylin, Lilly, and Sanofi. A.S. has received a research grant from Novo Nordisk for the LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) study as principal investigator for one of the study sites. D.C.G. served as a member of an operations committee for a clinical trial of a glucose-lowering medication marketed by Merck, a member of a DSMB for a clinical trial of a glucose-lowering medication marketed by Takeda, and a presenter at a continuing medical education meeting sponsored by Merck during the period of the research covered by this study. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. All authors participated in acquiring and interpreting the data. K.L.M., P.J.O., and D.C.G. wrote the manuscript. T.M.M. and G.W.E. completed the statistical analysis. J.B.B., R.M.C., W.C.C., J.A.C., H.C.G., R.H.G., E.W.L., K.M.V.N., M.C.R., and A.S. revised the manuscript and approved the final draft. K.L.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT00000620, (clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-2334/-/DC1.
References
- 1.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993;16:434–444 [DOI] [PubMed] [Google Scholar]
- 2.Kearney PM, Blackwell L, Collins R, et al. Cholesterol Treatment Trialists’ (CTT) Collaborators Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008;371:117–125 [DOI] [PubMed] [Google Scholar]
- 3.Turnbull F, Neal B, Algert C, et al. Blood Pressure Lowering Treatment Trialists’ Collaboration Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med 2005;165:1410–1419 [DOI] [PubMed] [Google Scholar]
- 4.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 5.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580–591 [DOI] [PubMed] [Google Scholar]
- 6.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–393 [DOI] [PubMed] [Google Scholar]
- 7.Goff DC, Jr, Gerstein HC, Ginsberg HN, et al. ACCORD Study Group Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: current knowledge and rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007;99(Supplement 1):4i–20i [DOI] [PubMed] [Google Scholar]
- 8.Buse JB, Bigger JT, Byington RP, et al. ACCORD Study Group Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol 2007;99(12A):21i–33i [DOI] [PubMed] [Google Scholar]
- 9.Ginsberg HN, Bonds DE, Lovato LC, et al. ACCORD Study Group Evolution of the lipid trial protocol of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007;99(12A):56i–67i [DOI] [PubMed] [Google Scholar]
- 10.Cushman WC, Grimm RH, Jr, Cutler JA, et al. ACCORD Study Group Rationale and design for the blood pressure intervention of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007;99(12A):44i–55i [DOI] [PubMed] [Google Scholar]
- 11.Gerstein HC, Miller ME, Genuth S, et al. ACCORD Study Group Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011;364:818–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cushman WC, Evans GW, Byington RP, et al. ACCORD Study Group Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginsberg HN, Elam MB, Lovato LC, et al. ACCORD Study Group Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362:1563–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ismail-Beigi F, Craven T, Banerji MA, et al. ACCORD trial group Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376:419–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317:703–713 [PMC free article] [PubMed] [Google Scholar]
- 17.Ismail-Beigi F, Craven TE, O’Connor PJ, et al. ACCORD Study Group Combined intensive blood pressure and glycemic control does not produce an additive benefit on microvascular outcomes in type 2 diabetic patients. Kidney Int 2012;81:586–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boden WE, Probstfield JL, Anderson T, et al. AIM-HIGH Investigators Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–2267 [DOI] [PubMed] [Google Scholar]
- 19.Clofibrate and niacin in coronary heart disease. JAMA 1975;231:360–381 [PubMed] [Google Scholar]
- 20.Scirica BM, Bhatt DL, Braunwald E, et al. SAVOR-TIMI 53 Steering Committee and Investigators Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–1326 [DOI] [PubMed] [Google Scholar]
- 21.White WB, Cannon CP, Heller SR, et al. EXAMINE Investigators Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–1335 [DOI] [PubMed] [Google Scholar]
- 22.Benavente OR, Coffey CS, Conwit R, et al. SPS3 Study Group Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial [published correction appears in Lancet 2013;382:506]. Lancet 2013;382:507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bangalore S, Kumar S, Lobach I, Messerli FH. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and Bayesian random-effects meta-analyses of randomized trials. Circulation 2011;123:2799–2810 [DOI] [PubMed]