Abstract
OBJECTIVE
We examined the effects of an intensive lifestyle intervention (ILI), compared with a diabetes support and education (DSE) control intervention, on long-term changes in depression symptoms, antidepressant medication (ADM) use, and health-related quality of life (HRQoL) in overweight/obese individuals with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Look AHEAD was a multisite randomized controlled trial of 5,145 overweight/obese participants assigned to ILI (designed to produce weight loss) or DSE and followed for a median of 9.6 years. The Beck Depression Inventory (BDI) was administered at baseline, annually at years 1–4, and again at year 8. Mean BDI scores and incidence of BDI scores ≥10, indicative of likely mild or greater depression, were examined. Annually through year 10, participants reported their ADM use and completed the Medical Outcomes Study Short Form 36 (SF-36) questionnaire, which yields physical component summary (PCS) and mental component summary (MCS) scores.
RESULTS
ILI significantly reduced the incidence of mild or greater depression symptoms (BDI scores ≥10) compared with DSE (hazard ratio [HR] = 0.85; 95% CI 0.75–0.97; P = 0.0145). Although SF-36 PCS scores worsened over time in both groups, ILI participants reported better physical function than DSE throughout the first 8 years (all P values <0.01). There were no significant differences between treatment arms in the proportion of participants who used ADMs or in SF-36 MCS scores.
CONCLUSIONS
ILI for overweight/obese patients with type 2 diabetes may reduce the risk of developing clinically significant symptoms of depression and preserve physical HRQoL. These findings should be considered when evaluating the potential benefits of ILIs.
Introduction
Individuals with type 2 diabetes are at high risk of depression (1) and have poor health-related quality of life (HRQoL), particularly concerning perceived general health and limitations in physical functioning (2,3). Depression in individuals with type 2 diabetes is associated with less active diabetes self-management (4), persistent hyperglycemia (5), higher rates of diabetes-related complications and mortality (3,5), and a 4.5-fold increase in health care expenditures (6).
Obesity is also associated with increased morbidity and mortality (7,8) and with higher levels of depression (9,10) and poorer HRQoL (11,12). Reducing the incidence of depression and improving HRQoL in obese persons with diabetes is an important clinical objective.
Behavioral weight loss interventions consistently induce reductions of 5% or more of initial weight in overweight/obese persons with type 2 diabetes (13), losses that are associated with initial improvements in the control of blood glucose, blood pressure, and lipid levels (14). Several studies also suggest that behavioral weight loss interventions facilitate short-term reductions in depression symptoms, as assessed by the Beck Depression Inventory (BDI) (15–18), as well as improvements in the physical health component of quality of life (19–22). However, only a few studies of weight loss and mood were conducted in persons with type 2 diabetes, and none followed participants for more than 4 years. Whether the effects observed in the short-term studies are sustained remains unknown. This question is of particular relevance because of lingering concerns about the possible adverse psychological effects of weight loss interventions, as reported in early weight reduction programs (23). The Look AHEAD (Action for Health in Diabetes) trial provides a unique opportunity to assess the effects of long-term intensive lifestyle intervention (ILI), designed to achieve weight loss, on depression symptoms and HRQoL.
Research Design and Methods
Participants
Look AHEAD was a randomized clinical trial that investigated the long-term health impact of an ILI in 5,145 overweight or obese adults with type 2 diabetes. The design and methods of this trial have been reported elsewhere (24), as have the baseline characteristics of the randomized cohort (25). The trial’s primary objective was to determine whether cardiovascular disease (CVD) morbidity and mortality would be reduced with an ILI designed to achieve weight reduction. As reported previously (26), the trial was stopped early on the basis of a futility analysis (when the median duration of follow-up was 9.6 years). No significant differences were observed between treatment arms on the primary outcome, a composite of death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for angina. The trial currently is continuing as an observational cohort study.
Look AHEAD participants were recruited at 16 clinical centers in the U.S. (25). Eligibility requirements included 45 to 76 years of age; self-reported type 2 diabetes mellitus verified by physician report, use of diabetes medication, or tested blood glucose levels; BMI ≥25 kg/m2 (≥27 kg/m2 in those taking insulin); hemoglobin A1c (HbA1c) levels <11% (<97 mmol/mol); systolic blood pressure <160 mmHg; diastolic blood pressure <110 mmHg; and triglyceride levels <600 mg/dL. Individuals with a current diagnosis of psychosis or bipolar disorder, or who had been hospitalized for depression in the past 6 months, were excluded.
Prior to randomization, participants completed 2 weeks of diet and exercise self-monitoring and successfully completed a maximal graded treadmill test to assess ability to exercise safely. All participants signed a consent form approved by their local institutional review board.
Interventions
Study participants were randomly assigned within each center to the ILI or to diabetes support and education (DSE), the comparison condition for this trial. As reported previously (14), the ILI was designed to induce ≥7% weight loss in the first year of the trial and to maintain the weight loss in subsequent years. ILI participants were prescribed a calorie goal of 1,200–1,800 kcal/day (based on initial weight), with <30% of total calories from fat (<10% from saturated fat) and ≥15% of total calories from protein. ILI participants were encouraged to engage in ≥175 min per week of moderate intensity exercise (e.g., brisk walking). The ILI incorporated behavioral strategies, including regular self-recording, to facilitate achievement of the above goals (13).
ILI participants were seen in a combination of individual and group sessions, weekly for the first 6 months and three times per month for the next 6 months. Sessions addressed behavioral adherence and ways to improve it. In all subsequent years, ILI participants were offered monthly in-person sessions. In addition, two to three times per year, they were invited to participate in a once-weekly 6–8 week group program designed to achieve a specific weight loss (e.g., 3–5 lbs.) or activity goal.
DSE participants were invited to three group sessions per year for the first 4 years. These sessions addressed general issues of diet, exercise, and social support using a standardized protocol. One group session per year was provided thereafter.
Outcome Measures
At baseline and study years 1, 2, 3, 4, and 8, participants completed the BDI-1A, a self-report depression symptom scale with well-documented psychometric properties (i.e., validity, reliability, and sensitivity to change) across a wide spectrum of clinical and nonclinical populations (15). The BDI-1A lists 21 symptoms, with responses scored from 0 to 3 for each item in ascending symptom severity, yielding a total score ranging from 0 to 63. Higher scores indicate more depression symptom burden. For this study, presence of elevated depression symptoms, indicating likely mild or greater depression, was defined as a BDI score ≥10 (15). (This study used the BDI-1A, rather than the BDI-II, which has different cut points for mild or greater depression [27].) The BDI-1A also may be scored, as it was in the current study, by separating the 14 items that assess principally cognitive and affective components of depression (e.g., negative mood and guilt) from the seven items that evaluate predominantly somatic components (e.g., appetite and sleep). Participants’ history of depression was assessed at baseline by questionnaire in response to the item: “Has a doctor or other health care provider ever said that you have depression?”
Twice annually for the first 4 years and then annually through year 10, participants completed the Medical Outcomes Study Short Form 36 (SF-36), a self-report HRQoL measure with well-documented psychometric properties across a wide range of clinical and nonclinical populations (28). The SF-36 provides eight subscale scores: general health perceptions; physical functioning; role limitations due to physical problems; bodily pain; mental health; role limitations due to emotional problems; vitality; and social functioning. Two summary norm-based T-scores (U.S. mean = 50, SD = 10) can also be derived from the SF-36: the physical component summary (PCS) score and mental component summary (MCS) score, with higher scores indicating more favorable HRQoL. The PCS score is derived from the first four subscales listed above, and the MCS from the last four subscales. Only the two summary scores were used in the present analyses, and only values from the annual assessments (at years 1–10) were examined.
Participants brought all prescription medications to their annual assessment visits, at which study staff recorded medications used (but not dosages). Antidepressant medications (ADMs) were identified using the Food and Drug Administration’s classification system.
Statistical Analyses
Analyses included all randomized participants according to intervention assignment (i.e., by intention to treat) and were performed using SAS version 9.3 (SAS Institute, Cary, NC). All data were censored at 14 September 2012, the day the intervention was stopped by the National Institutes of Health on the recommendation of the Data and Safety and Monitoring Board. At this time in the trial, all study participants had reached the 8-year assessment of mood and HRQoL. By contrast, fewer than 40% had reached the 10-year assessment of HRQoL when the study was halted, resulting in a substantially smaller sample of participants at this time, compared with year 8. (The same was true of the 9-year assessment of HRQoL.)
We examined the effect of the intervention on the incidence of likely mild or greater depression (BDI ≥10). Participants had to be free of the outcome at baseline to be included in the analysis. Hazard ratios (HRs) and 95% CIs from Cox proportional hazard models adjusting for clinic were compared between the two treatment groups using PROC PHREG. Time to event was defined as the time from randomization until the first occurrence of the outcome of interest. Proportional hazard assumptions were tested and met the criteria. Similar analyses were used to examine the potential incidence of worsening of depression symptoms (BDI score ≥19) in participants who began the trial already reporting mild depression (BDI score = 10–18).
Plots over time were constructed to assess the effect of the intervention on mean BDI, PCS, and MCS scores. Linear mixed longitudinal models controlling for clinic (the randomization stratification variable) were constructed using PROC MIXED to test the overall significance of treatment on these mean scores over time. Participants were treated as a random effect, and unstructured covariance structure techniques were used. Year-by-year differences in treatment effects were examined only when a significant treatment-by-time interaction existed.
Exploratory analyses (suggested during editorial review) examined the correlation among baseline BDI, PCS, and MCS scores to determine the extent to which the scales measured shared constructs. In addition, the ILI and DSE groups were compared on changes on the cognitive and somatic subscales of the BDI (15).
Results
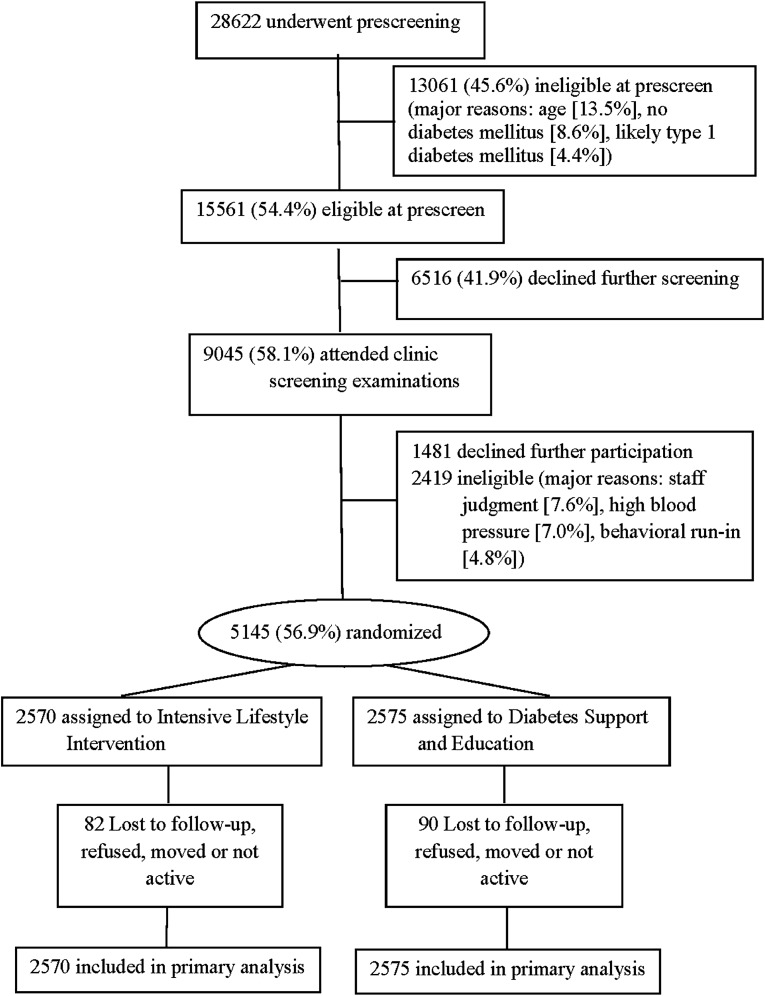
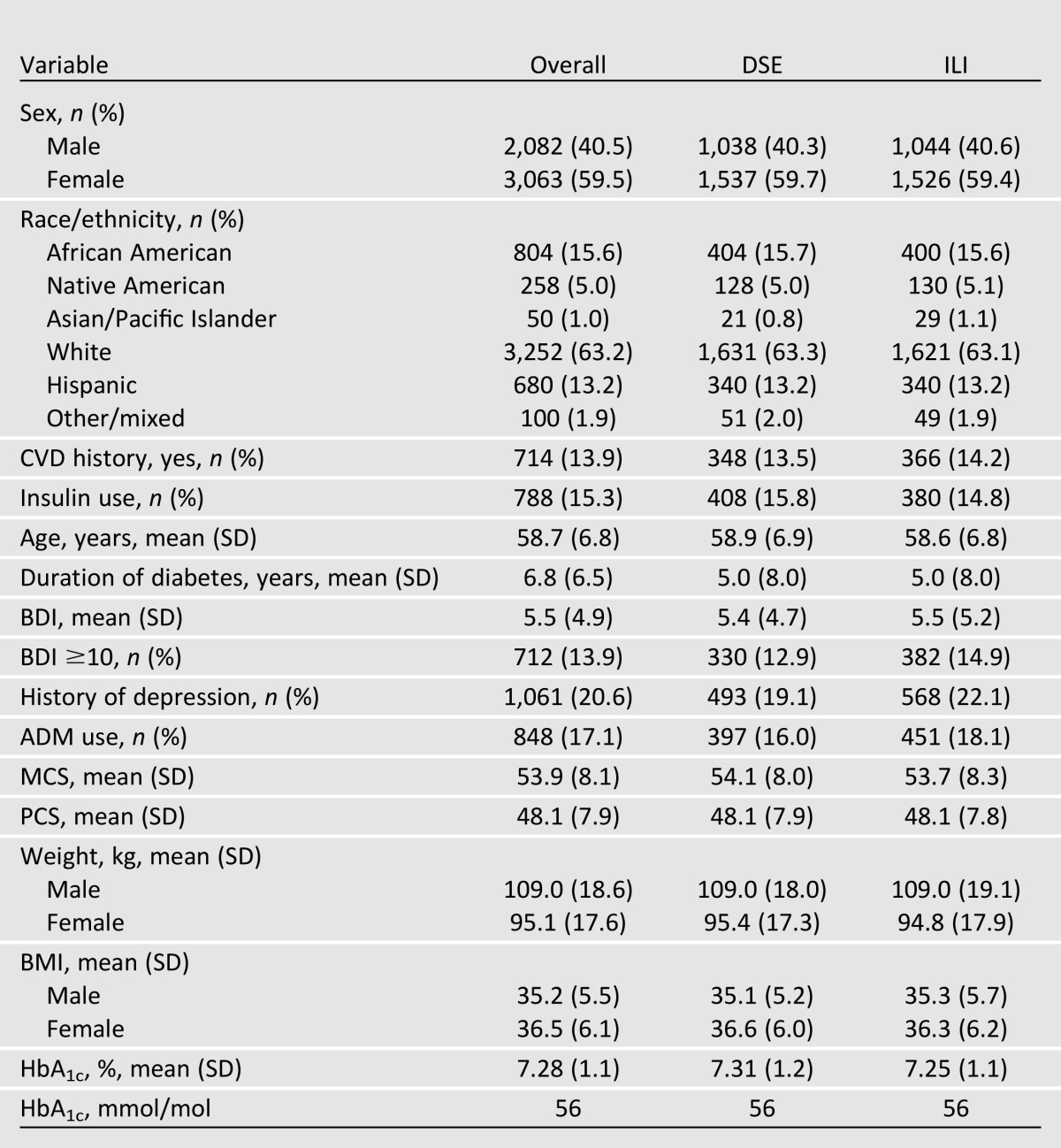
A total of 5,145 participants were randomly assigned to treatment conditions (2,570 to ILI and 2,575 to DSE) (see Fig. 1). Overall, 59.5% of participants were women, 63.2% were non-Hispanic white, and 14.0% reported a history of CVD at baseline. Participants had a mean (±SD) age of 58.7 (6.8) years, BMI of 36.0 (5.9) kg/m2, and duration of diabetes of 6.8 (6.5) years (see Table 1). At baseline, 18.2% of participants had a BDI ≥10, and 17.1% were taking ADMs, most frequently selective serotonin reuptake inhibitors, serotonin–norepinephrine reuptake inhibitors, and tricyclic antidepressants (in that order). At baseline, significantly (P < 0.0088) more ILI than DSE participants reported a history of depression (22.1 vs. 19.2%).
Figure 1.
CONSORT flow diagram.
Table 1.
Participants’ baseline characteristics
At year 1, ILI and DSE participants lost a mean of 8.6 and 0.7% of initial weight, respectively. Losses at end of study were 6.0 and 3.5%, respectively (both P values <0.0001).
Symptoms of Depression
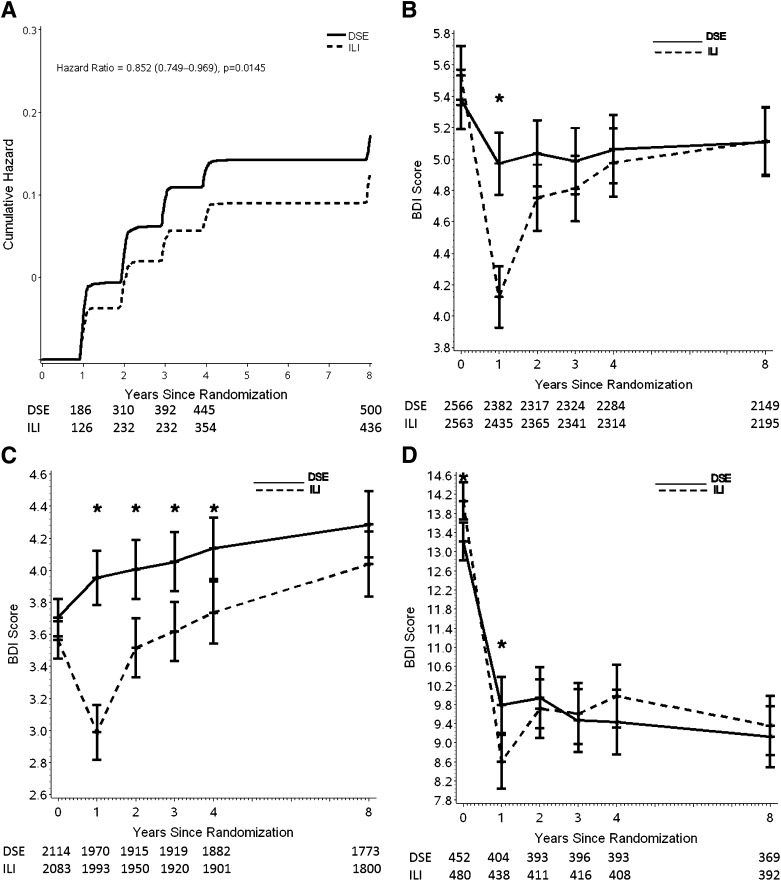
The ILI significantly reduced the incidence of mild or greater depression compared with DSE (HR = 0.85; 95% CI 0.75–0.97; P = 0.0145) (Fig. 2A). In the 81.8% of participants with baseline BDI scores in the nondepressed range (BDI scores <10), ILI participants were on average 15% less likely than those in DSE to progress to symptoms of likely mild or greater depression (BDI scores ≥10). (Adjusting for baseline history of depression, the HR was 0.82, 95% CI 0.732–0.935, and P = 0.0029.)
Figure 2.
(A) Cumulative HR (ILI versus DSE) for incidence of BDI ≥10 over 8 years in participants with BDI <10 at baseline. (B) Mean BDI score over 8 years by treatment arm for the full sample of participants. The sample size for DSE appears above the sample size for ILI for each year the BDI was administered. (C) Mean BDI score over 8 years by treatment arm for participants with a baseline BDI score <10 (indicative of no/minimal symptoms of depression). The sample size for DSE appears above the sample size for ILI for each year the BDI was administered. (D) Mean BDI score over 8 years by treatment arm for participants with a baseline BDI score ≥10 (indicative of mild or greater symptoms of depression). The sample size for DSE appears above the sample size for ILI for each year the BDI was administered. *, significant differences between groups (P < 0.005).
In participants who at baseline reported symptoms of mild depression (BDI score = 10–18; 403 in ILI, 410 in DSE), there were no significant differences between treatment arms in the proportion of individuals who progressed to moderate or greater symptoms of depression (BDI score ≥19; 79 participants [19.3%] in ILI, 71 [17.6%] in DSE; P value = 0.5441). In these same participants, a total of 337 (83.6%) in ILI and 342 (83.4%) in DSE had remission to a BDI score <10 (i.e., no/minimal depression symptoms) at some point during the follow-up, with no significant difference between treatment arms in time to first BDI <10 (HR = 1.11; 95% CI 0.96–1.30; P = 0.1718).
For the sample as a whole (n = 5,145), BDI scores averaged across the 8 years were significantly (P < 0.001) lower (i.e., better) in the ILI than the DSE group over the 8 years (Fig. 2B), resulting primarily from the larger reduction at year 1 in ILI than DSE participants (P < 0.001) (Fig. 2B). Subset analyses examined mean scores of participants who at baseline had no/minimal symptoms of depression (BDI score <10) and those with mild or greater symptoms (BDI score ≥10). In the nondepressed group (n = 4,197), Fig. 2C shows that there were significant differences between the ILI and DSE participants during each of the first 4 years, owing, in part, to small increases in mean BDI scores in the DSE group.
In participants who began the trial with mild or greater symptoms of depression (n = 932, which included a total of 119 participants with moderate or greater symptoms), mean BDI scores fell substantially in both groups the first year, though significantly (P = 0.0045) more in ILI than DSE (Fig. 2D). Thereafter, mean scores for both treatment arms varied by 1 to 1.5 points but generally remained in the nondepressed range (BDI score <10), with no significant differences between ILI and DSE participants in this subgroup.
Exploratory analyses of the BDI cognitive and somatic subscales were conducted using the full sample (n = 5,145). At year 1, ILI participants reported significantly greater reductions in both cognitive and somatic symptoms of depression than their DSE counterparts. The mean (SEM) difference in change between groups was 0.30 (0.09) on the cognitive subscale and 0.31 (0.08) on the somatic subscale (P < 0.05 for each comparison). At years 2, 3, 4, and 8, ILI participants reported significantly (P < 0.05 for all comparisons) greater reductions than DSE participants on the BDI somatic subscale (mean differences between groups ranging from 0.24 to 0.46) but not on the cognitive subscale (mean differences ranging from −0.01 to 0.05).
ADM Use
There were no significant differences between treatment arms in ADM use over the 10-year follow-up (P = 0.09). Across the two arms (n = 5,145), a total of 3,130 (64.3%) participants did not use ADMs at baseline or at any time during follow-up. By contrast, 773 (15.9%) took ADMs at baseline and continued to do so during follow-up. (Supplementary Fig. 1A presents these data by treatment arm.) A total of 897 participants (18.5%) initiated ADM use at some point after their baseline assessment (Supplementary Fig. 1B). There was no difference between the ILI and DSE groups in incident ADM use (HR = 1.08; 95% CI 0.95–1.23; P = 0.2316). Fifty-eight (1.2%) individuals who used ADMs at baseline permanently discontinued them within the first 4 years (32 ILI, 26 DSE participants) of randomization. There also were no significant differences at any time between the ILI and DSE groups in the mean number of ADMs used (P = 0.1472) (Supplementary Fig. 1C).
Quality of Life
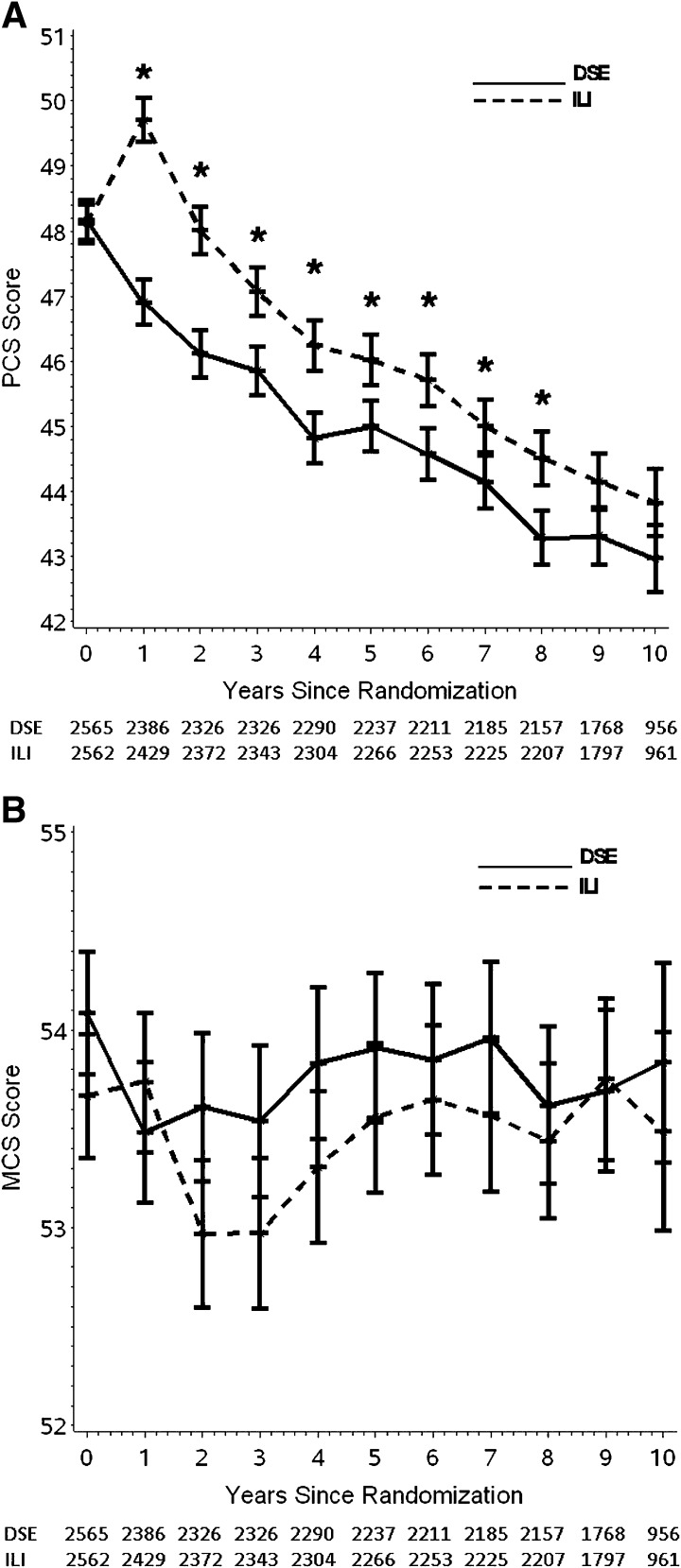
PCS scores declined (i.e., worsened) in both treatment arms over the 10-year follow-up. However, PCS scores remained significantly higher (i.e., better) in the ILI than the DSE arm over the decade (mean difference between groups = 0.93; SE = 0.2; P < 0.001) (Fig. 3A). Differences were statistically significant (P < 0.01 for all comparisons) at every year through the first 8 years of follow-up. During these 8 years, the PCS scores in ILI were 3.2% higher than those in DSE. In contrast, there were no significant differences between treatment arms in SF-36 MCS scores over the 10 years (P = 0.361; Fig. 3B).
Figure 3.
(A) Mean PCS scores over 10 years by treatment arm. The sample size for DSE appears the above sample size for ILI. *, significant differences between groups (P < 0.005). (B) Mean MCS scores over 10 years by treatment arm. The sample size for DSE appears above the sample size for ILI.
Exploratory analyses showed that baseline BDI scores correlated moderately with baseline PCS (r = 0.34; P < 0.0001) and MCS (r = 0.49; P < 0.0001) scores, with a correlation of r = 0.03 (P < 0.07) between the latter two measures. The correlation between the BDI somatic subscale and the PCS (r = 0.39; P < 0.0001), and that between the BDI cognitive subscale and the MCS (r = 0.52; P < 0.0001), increased slightly as compared with the full BDI.
Conclusions
This study found that a lifestyle intervention, designed to induce weight loss, reduced the risk of progressing to mild or greater symptoms of depression by an average of 15% over 8 years of follow-up, relative to DSE. The present findings confirm the results of previous studies of shorter duration (16–18,20) and provide the strongest evidence to date that ILI protects overweight/obese individuals from depression rather than precipitating it (23). The ILI’s benefit was not attributable to differential use of ADMs in the two treatment arms during the course of the trial.
In addition to its protective effects in nondepressed individuals, the ILI did not appear to harm individuals who had depression symptoms at the trial’s outset. Subgroup analyses showed that in participants who at baseline had mild depression symptoms (BDI score of 10–18), the lifestyle intervention did not increase the risk of progressing to moderate or greater depression symptoms (BDI score ≥19). Instead, at the end of the first year, ILI participants who began the trial with mild or greater depression symptoms (BDI score >10) experienced substantial improvements in mood, with reductions in mean BDI scores from 14.1 to 8.8. Part of the improvement likely was attributable to the spontaneous remission of depression, typically observed in 3 to 12 months (29). A similar (though more modest) pattern of improvement was observed the first year in comparable DSE participants, in whom mean BDI scores fell from 13.2 to 9.6.
The ILI’s prevention of mild or greater depression symptoms has important public health implications, particularly for people with type 2 diabetes. According to the World Health Organization, unipolar depression was the third most important cause of disease burden worldwide in 2004 and the most important in middle- and high-income countries (30). Depression is more prevalent among people with diabetes than it is in the general population. A study that included national samples reported that 6.1% of individuals without diabetes, and 9.3% of those with the condition, were currently depressed (5). The combination of depression and diabetes leads to negative effects on self-care behaviors (31) and glycemic control (4) and ultimately increases morbidity (5), mortality (5), and health care costs (6). Reducing the risk of developing depression over 8 years in individuals with diabetes could thus have an important health impact.
A second principal finding of this study is that participating in ILI may mitigate the effects of aging on physical HRQoL. Epidemiological studies have consistently reported a decline in SF-36 PCS scores with age (32–34). None of these studies involved follow-up of >5 years, and none was restricted to overweight/obese individuals or to those with type 2 diabetes. The current study, which followed participants for a median of 9.6 years, also found that PCS scores declined (i.e., worsened) in both treatment arms. However, participants in the ILI arm experienced significantly less decline than those in DSE over the course of the study and at every year of follow-up through year 8. This ILI advantage is consistent with the findings of other lifestyle interventions of shorter duration in overweight/obese adults (20–22). The lifestyle intervention, for example, in the Diabetes Prevention Program resulted in a mean 1.3 point increase in the PCS score at 1 year, as compared with a 0.04 decline in the placebo group.
Some investigators have suggested that people perceive a 3% difference in HRQoL scores (e.g., 1 to 1.5 T-score points on PCS) as beneficial or deleterious (35). By this criterion, the ILI, compared with DSE, produced a perceptible improvement in physical HRQoL over the first 8 years of follow-up. Other health professionals (36–38), however, have proposed that a 3 to 5 T-score point change on the PCS is required to be considered clinically meaningful, a magnitude which the ILI group only approached at year 1.
Slowing age-related decline in physical HRQoL has important public health implications, especially for individuals with diabetes. HRQoL is increasingly recognized as an essential measure of health status, especially in chronic diseases. An analysis of 118 studies found that SF-36 PCS scores were lower in individuals with type 2 diabetes compared with U.S. population norms (39), suggesting that interventions that mitigate age-related decline in physical functioning may have substantial benefits for those with type 2 diabetes.
The current study found no significant difference between the treatment and comparison arms in changes in mean MCS scores over the course of the study, a finding consistent with that from several similar trials (20,22,38). We examined the mental health subscale of the MCS to see if there were statistically significant treatment effects for this subscale (i.e., results more similar to those for BDI score; we found no such effects). The SF-36 mental health subscale is more general than the BDI, assessing mental health issues other than depression (e.g., anxiety) (30). This view is supported by the moderate correlation (r = 0.49) between the MCS and BDI observed in the current study and suggests that the mental health benefits of the ILI intervention were specific to protection from developing significant symptoms of depression.
We similarly observed that the baseline PCS score correlated only moderately with both the full BDI (r = 0.34) and the BDI somatic subscale (r = 0.39). While the PCS and BDI somatic subscale assess some similar domains (e.g., worry about health), the PCS includes numerous items on specific physical abilities (e.g., walking stairs) and bodily pain, which the BDI does not include. Conversely, the BDI somatic subscale evaluates complaints about sleep, appetite, appearance, and sexual function—hallmarks of depression—which are not assessed by the PCS. While the ILI participants reported significantly greater improvements on both the PCS and the BDI somatic subscale at all assessments during the first 8 years, we believe that the two measures captured different aspects of participants’ psychosocial and physical function (as also suggested by the scales’ shared variance of only 15.2%). ILI as compared with DSE participants reported greater reductions on the BDI cognitive subscale only at year 1, the time when the ILI group experienced its greatest improvement on the full BDI score (see Fig. 2B).
Several aspects of the lifestyle intervention may have contributed to the reduced risk of depression and improved HRQoL observed in this study. These include the greater initial weight losses and improvements in cardiorespiratory fitness achieved by ILI versus DSE participants (25). ILI participants also received extensive group and individual support that could have protected them from depression, as could their improvements in diabetes control and cardiovascular risk factors, which were generally greater in ILI than DSE participants during most of the trial (14,26). A separate paper will examine the factors that potentially mediated the reported improvements in depression and physical quality of life, as well as explore the relationships between initial depression level, changes in depression over time, and subsequent weight change. As reported previously, among ILI participants who began the study free of symptoms of depression (BDI score <10), those who experienced incident depression at 1 year lost significantly less weight at this time than their ILI counterparts who remained free of a mood disorder (mean losses of 4.6 vs. 9.0% of initial weight, respectively). By contrast, ILI participants who began the study with mild or greater symptoms of depression (BDI score >10) lost only marginally (though significantly) less weight at 1 year than ILI participants who were free of baseline depression (mean losses of 7.8 vs. 8.7%, respectively) (17).
Strengths of the current study include its large racially/ethnically diverse population, long duration of intervention, excellent participant retention, and use of well-validated questionnaires. Study limitations include that all participants had type 2 diabetes, potentially limiting the generalizability of our findings to populations without diabetes (although our results are similar to those observed in earlier trials of nondiabetic individuals) (18). Participants also generally had average to good mental health, as indicated by their BDI and MCS scores, thus raising questions about the generalizability of the findings to populations with a greater mental health burden. Our study would have been improved by the use of a structured clinical interview to evaluate depression (which also was conducted at all annual assessment visits). Alternatively, we could have administered the Patient Health Questionnaire-9 (40), which, unlike the BDI-1A, evaluates multiple domains needed to diagnosis major depressive disorder according to recognized criteria (29). Other study limitations included our not determining the dose of ADMs used and not identifying reasons other than depression that participants may have taken ADMs (e.g., anxiety, neuropathy). In addition, quality of life was assessed in fewer than 40% of participants at year 10, the approximate percentage of persons who, based on their date of enrollment in the trial, had been eligible to complete this assessment when the intervention was stopped in September 2012.
In summary, participation in an ILI, designed to produce weight loss, protected overweight/obese individuals with type 2 diabetes from developing mild or greater symptoms of depression, as well as reductions in HRQoL. Clinicians may want to take these findings into account when evaluating the potential benefits of behavioral weight loss treatment.
Article Information
Acknowledgments. The authors are Richard R. Rubin, Thomas A. Wadden, Judy L. Bahnson, George L. Blackburn, Frederick L. Brancati, George A. Bray, Mace Coday, Scott J. Crow, Jeffrey M. Curtis, Gareth Dutton, Caitlin Egan, Mary Evans, Lin Ewing, Lucy Faulconbridge, John Foreyt, Sarah A. Gaussoin, Edward W. Gregg, Helen P. Hazuda, James O. Hill, Edward S. Horton, Van S. Hubbard, John M. Jakicic, Robert W. Jeffery, Karen C. Johnson, Steven E. Kahn, William C. Knowler, Wei Lang, Cora E. Lewis, Maria G. Montez, Anne Murillo, David M. Nathan, Jennifer Patricio, Anne Peters, Xavier Pi-Sunyer, Henry Pownall, W. Jack Rejeski, Renate H. Rosenthal, Valerie Ruelas, Katie Toledo, Brent Van Dorsten, Mara Vitolins, Donald Williamson, Rena R. Wing, Susan Z. Yanovski, and Ping Zhang. The authors thank Patricia S. Hong of the University of Pennsylvania, Center for Weight and Eating Disorders, for her editorial assistance in preparing the manuscript. Richard R. Rubin wrote the initial drafts of this article. This publication is dedicated to his memory.
Funding. This study was supported by the Department of Health and Human Services through the following cooperative agreements from the National Institutes of Health: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. The following federal agencies have contributed support: National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; National Institutes of Health Office of Research on Women’s Health; and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service provided personnel, medical oversight, and use of facilities. Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719), the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066), the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520), the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140), the University of Pittsburgh General Clinical Research Center (M01RR000056), the Clinical Translational Research Center funded by the Clinical and Translational Science Award (UL1 RR 024153) and a National Institutes of Health grant (DK 046204), the Veterans Affairs Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs, and the Frederic C. Bartter General Clinical Research Center (M01RR01346). Some of the information contained herein was derived from data provided by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene. Federal support was provided by the National Institute of Diabetes and Digestive and Kidney Diseases (M.E., Barbara Harrison, V.S.H., and S.Z.Y.); the National Heart, Lung, and Blood Institute (to Lawton S. Cooper, Peter Kaufman, and Mario Stylianou); and the Centers for Disease Control and Prevention (to E.W.G. and P.Z.).
Duality of Interest. T.A.W. serves on advisory boards for Novo Nordisk and Orexigen and is a consultant to Boehringer Ingelheim. G.A.B. is a consultant to Herbalife Nutrition Institute, Jason Pharmaceuticals, and Theracos Pharmaceuticals. H.P.H. received honoraria and speaker fees from the Texas Association of Health-System Pharmacists. J.O.H. serves on advisory boards for McDonalds, Nestle, and Retrofit and is a consultant to Novo Nordisk. J.M.J. serves on the advisory board for Alere Wellbeing and has received honoraria and speaker fees from Jenny Craig, Nestle Nutrition Institute, Calorie Control Council, and Kaiser Permanente. A.P. is a consultant to Lilly Pharmaceuticals, Amylin, Janssen, AstraZeneca, and BMS and has received honoraria and speaker fees from Novo Nordisk, Takeda, AstraZeneca/BMS, Sanofi, and Amylin. The following organizations committed to make major contributions to Look AHEAD: FedEx Corporation, Health Management Resources, LifeScan Inc. (a Johnson & Johnson Company), OPTIFAST of Nestle HealthCare Nutrition Inc., Hoffmann-La Roche Inc., Abbott Nutrition, and Slim-Fast Brand of Unilever North America. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. R.R.R., T.A.W., S.A.G., H.P.H., A.P., B.V.D., R.R.W., S.Z.Y., and P.Z. were responsible for study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. J.L.B., G.L.B., F.L.B., G.A.B., M.C., S.J.C., J.M.C., G.D., C.E., M.E., L.E., L.F., J.F., E.W.G., J.O.H., E.S.H., V.S.H., J.M.J., R.W.J., K.C.J., S.E.K., W.C.K., W.L., C.E.L., M.G.M., A.M., D.M.N., J.P., X.P.-S., H.P., W.J.R., R.H.R., V.R., K.T., and D.W. were responsible for acquisition of data and critical revision of the manuscript for important intellectual content. T.A.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT00017953, clinicaltrials.gov.
A slide set summarizing this article is available online.
*The study’s authors are listed in the Acknowledgments section, and members of the Look AHEAD Research Group are listed in the Supplementary Data online.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-1928/-/DC1.
The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the Indian Health Service or other funding sources.
References
- 1.Egede LE, Zheng D. Independent factors associated with major depressive disorder in a national sample of individuals with diabetes. Diabetes Care 2003;26:104–111 [DOI] [PubMed] [Google Scholar]
- 2.Goldney RD, Phillips PJ, Fisher LJ, Wilson DH. Diabetes, depression, and quality of life: a population study. Diabetes Care 2004;27:1066–1070 [DOI] [PubMed] [Google Scholar]
- 3.Manuel DG, Schultz SE. Health-related quality of life and health-adjusted life expectancy of people with diabetes in Ontario, Canada, 1996-1997. Diabetes Care 2004;27:407–414 [DOI] [PubMed] [Google Scholar]
- 4.Lustman PJ, Clouse RE. Depression in diabetic patients: the relationship between mood and glycemic control. J Diabetes Complications 2005;19:113–122 [DOI] [PubMed] [Google Scholar]
- 5.Egede LE, Nietert PJ, Zheng D. Depression and all-cause and coronary heart disease mortality among adults with and without diabetes. Diabetes Care 2005;28:1339–1345 [DOI] [PubMed] [Google Scholar]
- 6.Egede LE, Zheng D, Simpson K. Comorbid depression is associated with increased health care use and expenditures in individuals with diabetes. Diabetes Care 2002;25:464–470 [DOI] [PubMed] [Google Scholar]
- 7.Haslam DW, James WP. Obesity. Lancet 2005;366:1197–1209 [DOI] [PubMed] [Google Scholar]
- 8.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA 2005;293:1861–1867 [DOI] [PubMed] [Google Scholar]
- 9.Onyike CU, Crum RM, Lee HB, Lyketsos CG, Eaton WW. Is obesity associated with major depression? Results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol 2003;158:1139–1147 [DOI] [PubMed] [Google Scholar]
- 10.Strine TW, Mokdad AH, Dube SR, et al. The association of depression and anxiety with obesity and unhealthy behaviors among community-dwelling US adults. Gen Hosp Psychiatry 2008;30:127–137 [DOI] [PubMed] [Google Scholar]
- 11.Jia H, Lubetkin EI. The impact of obesity on health-related quality-of-life in the general adult US population. J Public Health (Oxf) 2005;27:156–164 [DOI] [PubMed] [Google Scholar]
- 12.Anandacoomarasamy A, Caterson ID, Leibman S, et al. Influence of BMI on health-related quality of life: comparison between an obese adult cohort and age-matched population norms. Obesity (Silver Spring) 2009;17:2114–2118 [DOI] [PubMed] [Google Scholar]
- 13.Wadden TA, West DS, Delahanty L, et al. Look AHEAD Research Group The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14:737–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wing RR, Lang W, Wadden TA, et al. Look AHEAD Research Group Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011;34:1481–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck AT, Steer RA. Manual for the Beck Depression Inventory. San Antonio, Psychological Corporation, 1987 [Google Scholar]
- 16.Wing RR, Blair E, Marcus M, Epstein LH, Harvey J. Year-long weight loss treatment for obese patients with type II diabetes: does including an intermittent very-low-calorie diet improve outcome? Am J Med 1994;97:354–362 [DOI] [PubMed] [Google Scholar]
- 17.Faulconbridge LF, Wadden TA, Rubin RR, et al. Look AHEAD Research Group One-year changes in symptoms of depression and weight in overweight/obese individuals with type 2 diabetes in the Look AHEAD study. Obesity (Silver Spring) 2012;20:783–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wadden TA, Vogt RA, Andersen RE, et al. Exercise in the treatment of obesity: effects of four interventions on body composition, resting energy expenditure, appetite, and mood. J Consult Clin Psychol 1997;65:269–277 [DOI] [PubMed] [Google Scholar]
- 19.Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, New England Medical Center, 1994 [Google Scholar]
- 20.Ackermann RT, Edelstein SL, Narayan KM, et al. Diabetes Prevention Program Research Group Changes in health state utilities with changes in body mass in the Diabetes Prevention Program. Obesity (Silver Spring) 2009;17:2176–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williamson DA, Rejeski J, Lang W, Van Dorsten B, Fabricatore AN, Toledo K, Look AHEAD Research Group Impact of a weight management program on health-related quality of life in overweight adults with type 2 diabetes. Arch Intern Med 2009;169:163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin RR, Peyrot M, Wang N-Y, et al. Patient-reported outcomes in the practice-based opportunities for weight reduction (POWER) trial. Qual Life Res 2013;22:2389–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Task Force on the Prevention and Treatment of Obesity. Dieting and the development of eating disorders in overweight and obese adults. Arch Intern Med 2000;160:2581–2589 [DOI] [PubMed] [Google Scholar]
- 24.Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD Research Group Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003;24:610–628 [DOI] [PubMed] [Google Scholar]
- 25.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Look AHEAD Research Group Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 2007;30:1374–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wing RR, Bolin P, Brancati FL, et al. Look AHEAD Research Group Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, Psychological Corporation, 1996 [Google Scholar]
- 28.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care 1994;32:40–66 [DOI] [PubMed] [Google Scholar]
- 29.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed.. Washington, DC, American Psychiatric Press, 2013 [Google Scholar]
- 30.World Health Organization The Global Burden of Disease: 2004 Update. Geneva, WHO Press, 2008 [Google Scholar]
- 31.Gonzalez JS, Peyrot M, McCarl LA, et al. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care 2008;31:2398–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ware JE, Jr, Bayliss MS, Rogers WH, Kosinski M, Tarlov AR. Differences in 4-year health outcomes for elderly and poor, chronically ill patients treated in HMO and fee-for-service systems. Results from the Medical Outcomes Study. JAMA 1996;276:1039–1047 [PubMed] [Google Scholar]
- 33.Hopman WM, Berger C, Joseph L, et al. CaMos Research Group The natural progression of health-related quality of life: results of a five-year prospective study of SF-36 scores in a normative population. Qual Life Res 2006;15:527–536 [DOI] [PubMed] [Google Scholar]
- 34.Der-Martirosian C, Kritz-Silverstein D, Barrett-Connor E. Five-year stability in associations of health-related quality of life measures in community-dwelling older adults: the Rancho Bernardo Study. Qual Life Res 2010;19:1333–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407–415 [DOI] [PubMed] [Google Scholar]
- 36.Stewart AL, Greenfield S, Hays RD, et al. Functional status and well-being of patients with chronic conditions. Results from the Medical Outcomes Study. JAMA 1989;262:907–913 [PubMed] [Google Scholar]
- 37.Wyrwich KW, Tierney WM, Babu AN, Kroenke K, Wolinsky FD. A comparison of clinically important differences in health-related quality of life for patients with chronic lung disease, asthma, or heart disease. Health Serv Res 2005;40:577–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warkentin LM, Das D, Majumdar SR, Johnson JA, Padwal RS. The effect of weight loss on health-related quality of life: systematic review and meta-analysis of randomized trials. Obes Rev 2013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Norris SL, McNally TK, Zhang X, et al. Published norms underestimate the health-related quality of life among persons with type 2 diabetes. J Clin Epidemiol 2011;64:358–365 [DOI] [PubMed] [Google Scholar]
- 40.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]