Abstract
Background
Late-life depression is associated with white matter hyperintense lesions (WMLs) occurring in specific fiber tracts. In this study, we sought to determine if greater WML severity in the cingulum bundle or uncinate fasciculus was associated with poor short-term antidepressant response.
Methods
Eleven depressed elders completed a baseline cranial 3T MRI and received antidepressant treatment following a medication algorithm. MRIs were analyzed to measure the fraction of each fiber tract’s volume occupied by WMLs. Statistical analyses examined the effect of dichotomized fiber tract WML severity on three- and six-month depression severity after controlling for age and baseline depression severity.
Results
Greater WML severity in the left hemispheric cingulum bundle adjacent to the hippocampus was associated with greater post-treatment depression severity at three- (F1,7=6.42, p=0.0390) and six-month assessments (F1,5=9.62, p=0.0268). Other fiber tract WML measures were not significantly associated with outcomes.
Limitations
The study had a small sample size and analyses were limited to only a priori fiber tracts.
Conclusions
This pilot study supports the hypothesis that focal damage to the cingulum bundle may contribute to poor short-term antidepressant response. These findings warrant further investigation with a larger, more definitive study.
Keywords: Geriatrics, depression, antidepressants, hyperintense lesions, vascular disease, MRI, treatment response 1
INTRODUCTION
Late-life depression (LLD) is associated with greater comorbid vascular disease, characterized on MRI as white matter hyperintense lesions (WMLs) (Arnone et al., 2012; Taylor et al., 2005). As previously reviewed (Taylor et al., 2013a), WMLs are often more severe in depressed elders but measures of cerebral WML severity are not consistently associated with poorer antidepressant responses. The use of global measures of total cerebral WML volume rather than more specific measures of WML location may explain this heterogeneity across studies. As proposed in the Disconnection Hypothesis of Vascular Depression (Taylor et al., 2013a), focal damage to specific white matter fiber tracts may be more important to the occurrence and persistence of LLD.
Several studies provide clues about fiber tracts where damage may create a predisposition to LLD. LLD is associated with increased WML severity in the cingulum bundle, uncinate fasciculus, and superior longitudinal fasciculus (Dalby et al., 2010; Sheline et al., 2008; Taylor et al., 2013b). Similar findings are reported in these tracts using diffusion tensor imaging to measure fiber tract structural integrity in both late-life (Sexton et al., 2012; Taylor et al., 2007) and midlife depression (Zhang et al., 2012). The cingulum bundle and uncinate fasciculus may be particularly important as they are well defined tracts that connect frontal, cingulate, parietal, and temporal regions implicated in mood disorders. However, it is unclear if structural connectivity deficits in these tracts are associated with antidepressant outcomes.
The purpose of this study was to determine if focal disruption of white matter fiber tracts by WMLs adversely affects short-term response to antidepressants. We a priori focused on the cingulum bundle and uncinate fasciculus based on past reports associating these well-defined tracts with LLD (Dalby et al., 2010; Sheline et al., 2008; Taylor et al., 2013b). We hypothesized that individuals with greater WML severity in these tracts would exhibit greater depression severity after three and six months of antidepressant treatment.
2. MATERIALS AND METHODS
2.1. Design and sample
Depressed subjects enrolled in the NIMH-sponsored Conte Center for the Neuroscience of Depression in Late-Life and the Neurocognitive Outcomes of Depression in the Elderly (NCODE) study at Duke University Medical Center. The Duke Institutional Review Board approved the study protocol and all participants provided written informed consent.
Participants were aged 60 years or older and met DSM-IV criteria for Major Depressive Disorder, single episode or recurrent. Diagnosis was based on the NIMH Diagnostic Interview Schedule (DIS) and confirmed by clinical interview with a geriatric psychiatrist. Exclusion criteria included: 1) other major psychiatric illnesses, including bipolar disorder; 2) substance abuse or dependence; 3) primary neurologic illnesses, including dementia; 4) Mini-Mental State Exam score < 25; and 5) contraindications for magnetic resonance imaging (MRI).
Data from these subjects have previously been included in a report of differences in WML severity across fiber tracts between 91 depressed and nondepressed elders (Taylor et al., 2013b). Only depressed elders are included in the current study, and additionally the MRI had to be acquired at baseline and participants had to be followed for at least three months. This resulted in a final sample of 11 subjects.
2.2. Treatment and Assessment
All participants received antidepressant treatment using the Duke STAGED approach (Steffens et al., 2002). This algorithm mimics “real world” treatment options, accounting for past treatments and current depression severity, rather than adhering to a rigid clinical trial design. Although sertraline is identified as the initial study drug, the antidepressant regimen differed across the sample based on depression severity, past treatments, medication tolerability, and response. No subjects received either psychotherapy or electroconvulsive therapy during the study period. Subjects were seen every three months and the Montgomery-Asberg Depression Rating Scale (MADRS) was used to measure depression severity. To examine short-term antidepressant response, for this report we focused on 3- and 6-month data.
2.3 MRI Acquisition and Image Analysis
Cranial MRI was performed using the eight-channel parallel imaging head coil on a 3T whole-body MRI system (Trio, Siemens Medical Systems, Malvern, PA). T1-weighted, T2-weighted, proton density, and fluid-attenuated inversion recovery (FLAIR) images were acquired. Full acquisition parameters have been previously reported (Taylor et al., 2013b).
These four acquisitions were used for an automated tissue segmentation process to identify white matter, gray matter, cerebrospinal fluid, and WMLs. As previously reported (Chang et al., 2011), this process uses a probability atlas to guide an iterative analysis to identify different tissue types by differences in intensity across the four acquisitions. WMLs are detected as “outliers” to the normal tissue distributions. As previously reported (Taylor et al., 2013b) the lesion maps arising from this segmentation were aligned with a probabilistic white matter tract atlas (S. Mori, Johns Hopkins Medical Institute). This allowed for calculation of the fraction of tract occupied by lesion, or the proportion of voxel within a tract consisting of lesion among all voxels projected as being within that tract.
2.4 Data Analysis
All analyses were conducted using SAS 9.3 (Cary, NC). Out of the 20 tracts identified by our method, we a priori focused on three bilateral tracts: the cingulum bundle adjacent to the cingulate gyrus (CgC), the cingulum bundle, hippocampus region (CgH), and the uncinate fasciculus (UF). We quantified the fraction of each tract occupied by WMLs and dichotomized these variables as “high” and “low” WML severity determined by a median split of the entire dataset of 91 subjects (Taylor et al., 2013b).
Primary analyses consisted of general linear models (PROC GLM) examining MADRS score at either 3- or 6-months as the dependent variable. Independent variables included the dichotomized median split of the fiber tract white matter lesion (ftWML) fraction, age, and baseline MADRS score. For models where the ftWML measure was significantly associated with 3- or 6-month MADRS score, we assessed the effect size as the semi-partial eta-squared statistic. To determine if any findings were specific to WMLs within the tract and not simply reflective of overall WML burden, a similar approach was to examine the relationship between total cerebral WML volume and treatment outcomes.
3. RESULTS
3.1. Demographics
The sample consisted of 11 depressed participants (6 women, 5 men) with a mean age of 64.6 years (SD = 4.4y, range 60–74y). They were cognitively intact, with a mean MMSE score of 29 (SD=1.8, range 24–30). Mean baseline depression severity by MADRS was 24.1 (SD = 10.5, range 16–44); after antidepressant treatment, the three-month mean MADRS score was 12.1 (SD = 8.1, range = 1–24). Most subjects received a serotonin reuptake inhibitor (sertraline = 6, citalopram = 2, fluoxetine = 1), with two subjects receiving venlafaxine.
3.2. WML severity and antidepressant outcomes
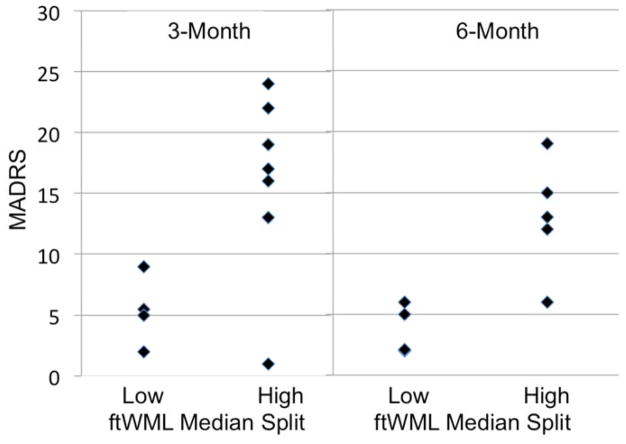
After controlling for age and baseline depression severity, only the left CgH tract was significantly associated with 3-month depression severity (Table 1). In this analysis, the 4 subjects with lower CgH WML severity all had 3-month MADRS scores of less than 10 while only 1 of 7 subjects with greater CgH severity had a 3-month MADRS less than 10 (Figures 1 and 2). This relationship exhibited a large effect size, with an eta-squared value of 0.47. None of the other 5 a priori defined regions were significantly associated with 3-month MADRS score. Similarly, we did not find a difference between dichotomized total cerebral WML severity and 3-month MADRS score.
Table 1.
MADRS score by Fiber-tract WML severity
| Region | Low WML severity | High WML severity | F value | p value |
|---|---|---|---|---|
| Three-Month | ||||
| CgC, left | 8.8 (7.0) | 14.8 (8.6) | F 1,7 = 1.61 | 0.2454 |
| CgC, right | 9.8 (7.7) | 13.4 (8.7) | F 1,7 = 0.42 | 0.5375 |
| CgH, left | 5.3 (2.9) | 16.0 (7.6) | F 1,7 = 6.42 | 0.0390 |
| CgH, right | 7.8 (7.6) | 14.6 (7.9) | F 1,7 = 2.25 | 0.1775 |
| UF, left | 8.8 (7.0) | 14.8 (8.6) | F 1,7 = 1.61 | 0.2454 |
| UF, right | 10.4 (6.8) | 15.0 (10.5) | F 1,7 = 0.90 | 0.3751 |
| Total cerebral WML | 9.4 (7.6) | 14.3 (8.6) | F 1,7 = 0.97 | 0.3575 |
| Six-Month | ||||
| CgC, left | 7.6 (6.6) | 10.5 (5.8) | F1, 5 = 0.39 | 0.5582 |
| CgC, right | 8.3 (7.4) | 9.4 (5.6) | F1, 5 = 0.03 | 0.8696 |
| CgH, left | 3.7 (2.1) | 13.0 (4.7) | F1, 5 = 9.62 | 0.0268 |
| CgH, right | 8.0 (7.5) | 9.6 (5.4) | F1, 5 = 0.21 | 0.6640 |
| UF, left | 7.6 (6.6) | 10.5 (5.8) | F1, 5 = 0.39 | 0.5582 |
| UF, right | 8.7 (6.7) | 9.5 (4.9) | F1, 5 = 0.01 | 0.9208 |
| Total cerebral WML | 9.4 (7.2) | 8.3 (5.2) | F1, 5 = 0.10 | 0.7674 |
Determination of low versus high WML severity based on a median split of WML measures within each tract based on the entire sample of 91 subjects (Taylor WD et al, Hum Brain Map, 2013). All models examined depression severity as measured by MADRS, while controlling for age and baseline depression severity. For three-month data, N = 11; for 6-month data, N = 9.
CgC = Cingulum bundle adjacent to cingulate gyrus; CgH = Cingulum bundle adjacent to hippocampus; UF = Uncinate Fasciculus
Figure 1.
Three- and six-month MADRS scores based on lower or higher white matter lesion severity in the left hemisphere cingulum bundle/hippocampus region.
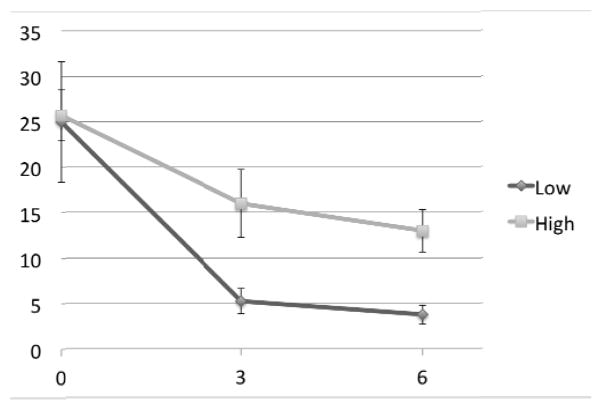
Figure 2.
Change in MADRS by left cingulum bundle white matter lesion severity Legend: Chart displays MADRS score (y-axis) at baseline (time 0), 3 months, and 6 months. Data presented as mean values for high and low white matter lesion severity in the left cingulum bundle/hippocampus region. Error bars reflect standard deviation.
Of the 11 subjects with 3-month data, 9 subjects had 6-month data, with a mean 6-month MADRS of 8.9 (SD = 6.1, range = 2–19). Similar to results examining the 3-month outcomes, only the left CgH WML severity was associated with 6-month outcome (Table 1) where subjects with lower CgH WML severity continued to have MADRS scores less than 10 (Figures 1 and 2). This relationship also exhibited a large effect size, with an eta-squared value of 0.61. There was not a significant relationship between dichotomized total cerebral WML severity and 6-month MADRS score.
DISCUSSION
As previously reviewed (Taylor et al., 2013a), the literature examining the relationship between cerebral WMLs and acute antidepressant response is mixed, with some studies finding that greater WML burden is associated with poor antidepressant response, while others did not find such an association. However, as proposed in the Disconnection Hypothesis of Vascular Depression (Taylor et al., 2013a), focal tract damage by WMLs may be more specifically associated with LLD or antidepressant outcomes than is global WML severity. To our knowledge, the current study is the first to find that greater WML severity in the portion of the cingulum bundle adjacent to the hippocampus is associated with poorer antidepressant response. Although limited by a small sample, our findings exhibited large effect sizes and were comparable at both assessment points. Neither total cerebral WML severity nor WML severity in other tracts were associated with three- or six-month response.
These data support the Disconnection Hypothesis by demonstrating that focal injury to the cingulum bundle has clinical consequences. Although our sample size is low, it is supported by previous studies implicating cingulum bundle pathology in depression (Taylor et al., 2013b; Zhang et al., 2012). However, the region of the cingulum bundle adjacent to the hippocampus identified in the current study is distinct from the region of the cingulum bundle we previously reported as exhibiting greater WML severity in LLD (Taylor et al., 2013b). This suggests that neural circuit damage that predisposes individuals to develop LLD may be different than neural circuit damage that adversely influences response to antidepressants.
Disruption of the cingulum bundle may potentially influence antidepressant response through two linked mechanisms. First, in early Alzheimer disease, disruption of the cingulum bundle in this region is highly correlated with posterior cingulate cortex hypometabolism and more broadly with dysfunction of the memory circuit of Papez (Villain et al., 2008). Although our sample did not exhibit dementia, it is possible that WML damage to the cingulum bundle may contribute to memory impairment, which is common in LLD and associated with poor antidepressant response (Sheline et al., 2010; Taylor et al., 2013a). Second, the hippocampus and posterior cingulate cortex are components of the posterior hub of the default mode network (Buckner et al., 2008). Function of this network is altered in depression (Sheline et al., 2009) and may normalize with an antidepressant response (Andreescu et al., 2013; Delaveau et al., 2011). It is possible that structural damage to the hippocampal region of the cingulum bundle may create a predisposition that reduces the likelihood of normalization of default mode network activity. Importantly, these mechanisms may be linked as regions of the default mode network exhibit changes in activity with episodic memory formation and retrieval (Chai et al., 2013; Sestieri et al., 2011). However, it is unclear why we found an association with the left but not right cingulum bundle.
The primary study limitation is its small sample. This reduced our power to detect differences in other tracts, so potentially damage to other fiber tracts may also adversely affect antidepressant response. Moreover, we would like to use these measures to predict antidepressant response. A larger, better-powered study with a validation sample is necessary to address these limitations. Additionally, participants were treated according to an antidepressant algorithm rather than through a standard clinical trial design. Although our approach mirrors “real world” treatment, focal neural circuit deficits may not equally affect response to antidepressants with different mechanisms of action. Finally, due to the small sample size, we could not additionally incorporate cognitive measures that predict acute antidepressant response (Taylor et al., 2013a).
We propose future research should further examine these questions in a larger, more definitive sample. The design could include antidepressants with different mechanisms of action, to determine if common circuitry deficits predict nonresponse to all antidepressants, or if there is specificity between circuit damage and response based on the drug’s mechanism of action. Such a “lesion model” examining antidepressant response could then inform similar studies examining how deficits in neural circuits affects response to antidepressants in a younger population.
Acknowledgments
Funding Source:
This project was supported by the National Institute of Mental Health (NIMH) grants R01 MH077745, R01 MH054846, K24 MH070027, and P50 MH60451
Footnotes
Preliminary data from this study were presentation at the 2013 Annual Meeting of the American Association for Geriatric Psychiatry in Los Angeles, CA.
Contributors
Dr. Taylor and Dr. Steffens designed the study and developed the hypotheses. Dr. Taylor and Mr. Kudra wrote the initial draft of the manuscript and Mr. Kudra managed the literature searches. Drs. Zhao and MacFall guided MRI acquisition and analysis of MRI data. Drs. Taylor, Steffens, and MacFall contributed to data interpretation. All authors have contributed to and approved the final manuscript.
The authors report no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreescu C, Tudorascu DL, Butters MA, Tamburo E, Patel M, Price J, Karp JF, Reynolds CF, 3rd, Aizenstein H. Resting state functional connectivity and treatment response in late-life depression. Psychiatry Res. 2013 doi: 10.1016/j.pscychresns.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, McIntosh AM, Ebmeier KP, Munafo MR, Anderson IM. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. Eur Neuropsychopharmacol. 2012;22:1–16. doi: 10.1016/j.euroneuro.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Ofen N, Gabrieli JD, Whitfield-Gabrieli S. Development of deactivation of the default-mode network during episodic memory formation. Neuroimage. 2013;84C:932–938. doi: 10.1016/j.neuroimage.2013.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Yu SC, McQuoid DR, Messer DF, Taylor WD, Singh K, Boyd BD, Krishnan KR, MacFall JR, Steffens DC, Payne ME. Reduction of dorsolateral prefrontal cortex gray matter in late-life depression. Psychiatry Res. 2011;193:1–6. doi: 10.1016/j.pscychresns.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby RB, Chakravarty MM, Ahdidan J, Sorensen L, Frandsen J, Jonsdottir KY, Tehrani E, Rosenberg R, Ostergaard L, Videbech P. Localization of white-matter lesions and effect of vascular risk factors in late-onset major depression. Psychol Med. 2010;40:1389–1399. doi: 10.1017/S0033291709991656. [DOI] [PubMed] [Google Scholar]
- Delaveau P, Jabourian M, Lemogne C, Guionnet S, Bergouignan L, Fossati P. Brain effects of antidepressants in major depression: a meta-analysis of emotional processing studies. J Affect Disord. 2011;130:66–74. doi: 10.1016/j.jad.2010.09.032. [DOI] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Romani GL, Shulman GL. Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J Neurosci. 2011;31:4407–4420. doi: 10.1523/JNEUROSCI.3335-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton CE, Le Masurier M, Allan CL, Jenkinson M, McDermott L, Kalu UG, Herrmann LL, Bradley KM, Mackay CE, Ebmeier KP. Magnetic resonance imaging in late-life depression: vascular and glucocorticoid cascade hypotheses. Br J Psychiatry. 2012;201:46–51. doi: 10.1192/bjp.bp.111.105361. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. Proc Natl Acad Sci USA. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Pieper CF, Barch DM, Welsh-Boehmer K, McKinstry RC, MacFall JR, D’Angelo G, Garcia KS, Gersing K, Wilkins C, Taylor W, Steffens DC, Krishnan RR, Doraiswamy PM. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67:277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Vaishnavi SN, Mintun MA, Barch DM, Epstein AA, Wilkins CH, Snyder AZ, Couture L, Schechtman K, McKinstry RC. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. Am J Psychiatry. 2008;165:524–532. doi: 10.1176/appi.ajp.2007.07010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens DC, McQuoid DR, Krishnan KRR. The Duke Somatic Treatment Algorithm for Geriatric Depression (STAGED) approach. Psychopharmacol Bull. 2002;36:58–68. [PubMed] [Google Scholar]
- Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: Mechanisms linking vascular disease with depression. Mol Psychiatry. 2013a doi: 10.1038/mp.2013.20. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Gerig G, Krishnan KR. Structural integrity of the uncinate fasciculus in geriatric depression: Relationship with age of onset. Neuropsychiatr Dis Treat. 2007;3:669–674. [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Payne ME, McQuoid DR, Steffens DC, Provenzale JM, Krishnan KR. Greater MRI lesion volumes in elderly depressed subjects than in control subjects. Psychiatry Res. 2005;139:1–7. doi: 10.1016/j.pscychresns.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Zhao Z, Ashley-Koch A, Payne ME, Steffens DC, Krishnan RR, Hauser E, MacFall JR. Fiber tract-specific white matter lesion severity Findings in late-life depression and by AGTR1 A1166C genotype. Hum Brain Mapp. 2013b;34:295–303. doi: 10.1002/hbm.21445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villain N, Desgranges B, Viader F, de la Sayette V, Mezenge F, Landeau B, Baron JC, Eustache F, Chetelat G. Relationships between hippocampal atrophy, white matter disruption, and gray matter hypometabolism in Alzheimer’s disease. J Neurosci. 2008;28:6174–6181. doi: 10.1523/JNEUROSCI.1392-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Leow A, Ajilore O, Lamar M, Yang S, Joseph J, Medina J, Zhan L, Kumar A. Quantitative tract-specific measures of uncinate and cingulum in major depression using diffusion tensor imaging. Neuropsychopharmacology. 2012;37:959–967. doi: 10.1038/npp.2011.279. [DOI] [PMC free article] [PubMed] [Google Scholar]