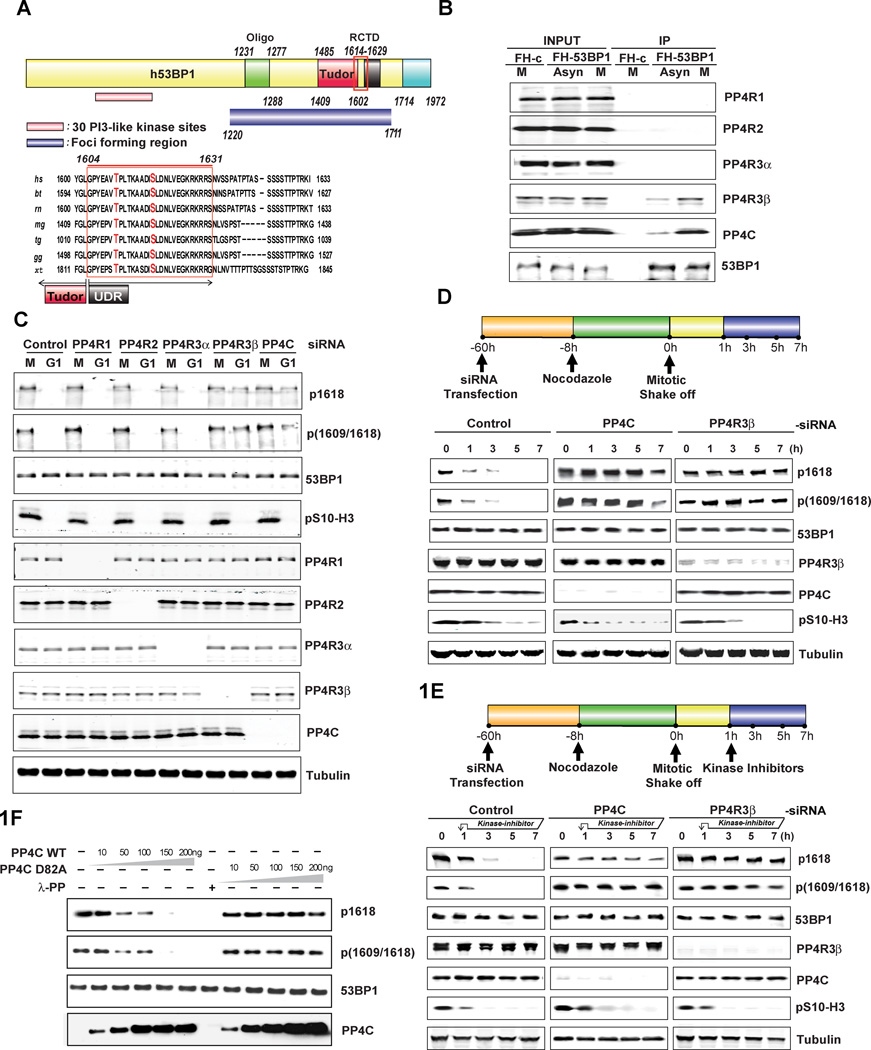
Figure 1. 53BP1 is a bonafide substrate of PP4C/PP4R3β.
A. Schematic representation of human 53BP1 and alignment of the region flanking T1609 and S1618 showing evolutionary conservation of this region. Sequence alignment was performed with ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2).
B. Interaction of 53BP1 with PP4C and PP4R3β. U2OS cells, stably expressing FH-Empty vector (FH-c) or FH-53BP1 were harvested after 8h synchronization in mitosis (M) using 100 ng/ml Nocodazole or left in an asynchronous state (Asyn). Whole cell extracts were immunoprecipitated with anti-FLAG agarose beads and analyzed by immunoblotting using indicated antibodies.
C. Impact of PP4C and PP4R3β silencing on 53BP1 phosphorylation. HeLa cells were transfected with indicated siRNAs against PP4 subunits for 60h and synchronized in mitosis with 100 ng/ml Nocodazole for 8h. Cells were released by mitotic shake-off into media without drug and harvested after 5h (G1 phase). Whole-cell lysates were probed with 53BP1 phospho-1618 (p1618) and 53BP1 phospho-1609/1618 (p1609–1618) antibodies. Total 53BP1 and tubulin were used as loading controls. Phospho-Ser10-histone H3 (pS10-H3) was used to indicate mitotic (M) cells. Cells were probed in parallel with antibodies against PP4R1, PP4R2, PP4R3α, PP4R3β, and PP4C to determine knockdown efficiency and specificity.
D. Kinetics of 53BP1 hyperphosphorylation in PP4C/PP4R3β silenced cells during transition from mitosis to G1 phase. Upper panel: Schematic to study kinetics of 53BP1 hyperphosphorylation. Lower panel: HeLa cells transfected with scrambled control, PP4C or PP4R3β siRNAs, synchronized in mitosis with 100 ng/ml Nocodazole, released by mitotic shake-off, and harvested at indicated time points for western blot analysis. Cell lysates were probed with indicated antibodies.
E. Inhibition of kinases PLK-1 and p38-MAPK in PP4C/PP4R3β silenced cells does not affect hyperphosphorylation of 53BP1 at T1609 and S1618. Upper panel: Schematic to study PLK-1 and p38-MAPK inhibition in PP4C/PP4R3β silenced cells. Lower panel: HeLa cells were transfected with indicated siRNAs and treated with Nocodazole as in Fig 1D. Kinase inhibitors against PLK-1 (BI2536, 20nM) and p38-MAPK (SB202190, 10µM) were added to the cells1h following release from mitosis. Cells were harvested at various time-points after mitotic release and lysates were probed with indicated antibodies.
F. Recombinant PP4C incubated with mitotic extracts shows a dose-dependent decrease in phosphorylation at T1609 and S1618 whereas the catalytically inactive PP4C D82A mutant protein fails to dephosphorylate even at high concentrations. Extract was also incubated with λ-protein phosphatase (PP) as a positive control.